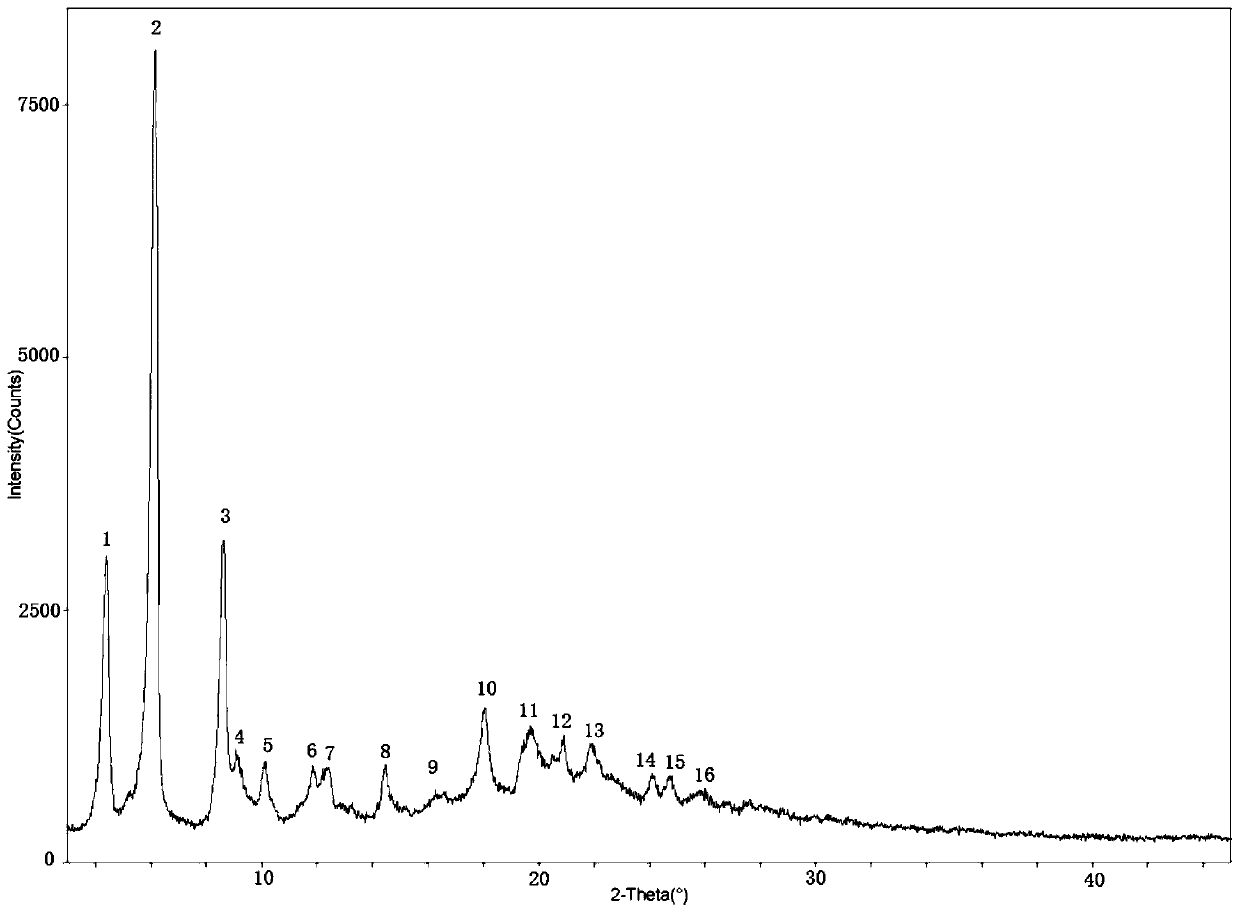
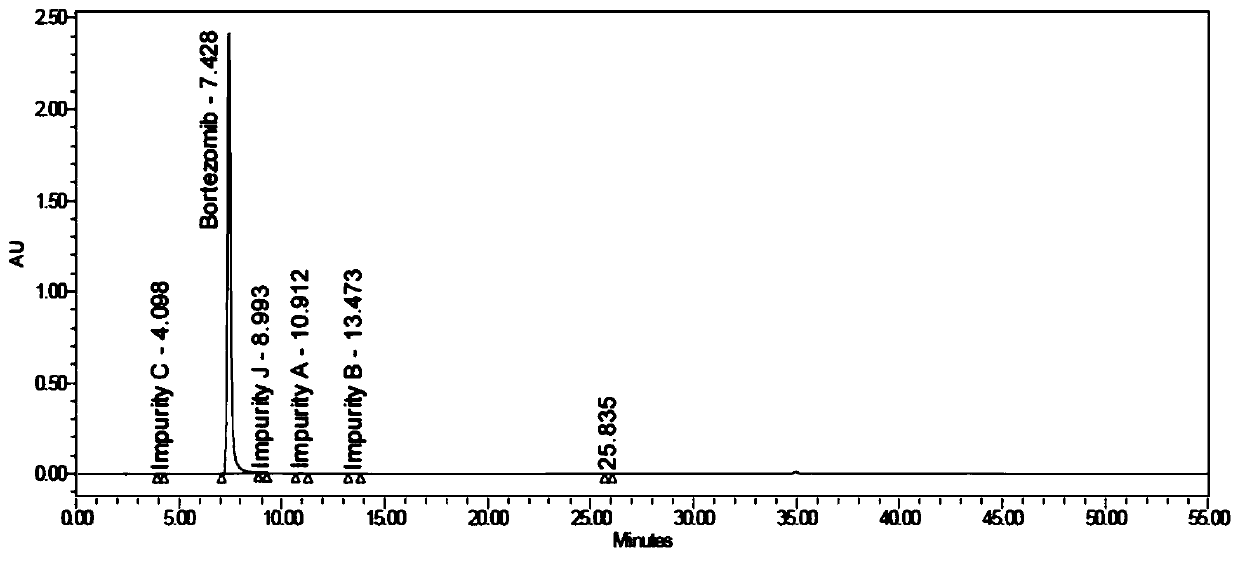
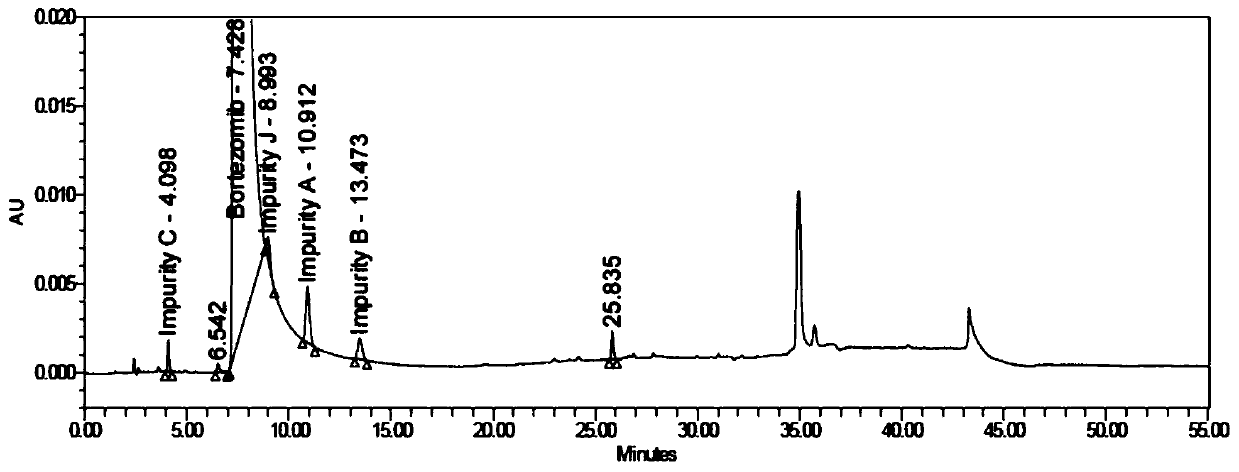
Bortezomib crystal form M, and preparation method and application thereof
A technology of bortezomib and crystal form, applied in the field of bortezomib crystal form M and its preparation, can solve the problems of low stability of bortezomib crystal form, unfavorable liquid preparation preparation, weak anti-oxidation ability, etc., and achieve clarity Good and visible foreign matter, long-term storage, less increase in impurities, and fast dissolution
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0048] A method for preparing bortezomib crystal form M, comprising the steps of:
[0049] Add the crude bortezomib to the test tube containing tetrahydrofuran according to the mass volume ratio of 0.3g / mL to obtain a tetrahydrofuran mixed solution, then open the test tube and place it in a beaker containing methyl tert-butyl ether and seal the beaker. The mass volume ratio of tezomib crude product to methyl tert-butyl ether is 0.04g / mL, the test tube mouth is higher than the height of methyl tert-butyl ether in the beaker, and methyl tert-butyl ether diffuses into the tetrahydrofuran mixed solution through the solvent, Crystallization at 25 ° C, that is.
Embodiment 2
[0051] A method for preparing bortezomib crystal form M, comprising the steps of:
[0052] Add the crude product of bortezomib to the test tube containing methyl tetrahydrofuran according to the mass volume ratio of 0.1g / mL to obtain a mixed solution of methyl tetrahydrofuran, then place the test tube mouth upwards and place it in a beaker filled with isopropyl ether and seal the beaker. The mass volume ratio of crude bortezomib to isopropyl ether is 0.02g / mL, the test tube mouth is higher than the height of the isopropyl ether in the beaker, the isopropyl ether diffuses into the mixed solution of methyl tetrahydrofuran through the solvent, and crystallizes at -10°C , that is.
Embodiment 3
[0054] A method for preparing bortezomib crystal form M, comprising the steps of:
[0055] The crude product of bortezomib was added into a three-necked flask filled with tetrahydrofuran under nitrogen protection according to the mass volume ratio of 0.2g / mL, and methyl tert-butyl ether was slowly added under stirring until the solid was precipitated and dissolved in a short while, and 3% (relative to bortezomib (mass fraction of rice crude product) seed crystal M (prepared by Example 1) and continue to stir, after obvious solid precipitation, continue to add methyl tert-butyl ether to the mass of bortezomib crude product and methyl tert-butyl ether The volume ratio is 0.04g / mL. Cool down to 15°C and stir for 4 hours, then filter, and wash the filter cake with a mixture of methyl tert-butyl ether / tetrahydrofuran (volume ratio 5:1) to obtain the obtained product.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com