Fungus-derived beta propeller-type recombinant phytase r-AoPhytase and expression strain and application thereof
A propeller-type, phytase technology, applied in the fields of molecular biology and genetic engineering, can solve the problems of low specific enzyme activity and unsuitable feed processing technology, and achieve the effect of promoting release
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0060] Example 1: AoPhytase codon optimization and its recombinant expression in Pichia pastoris
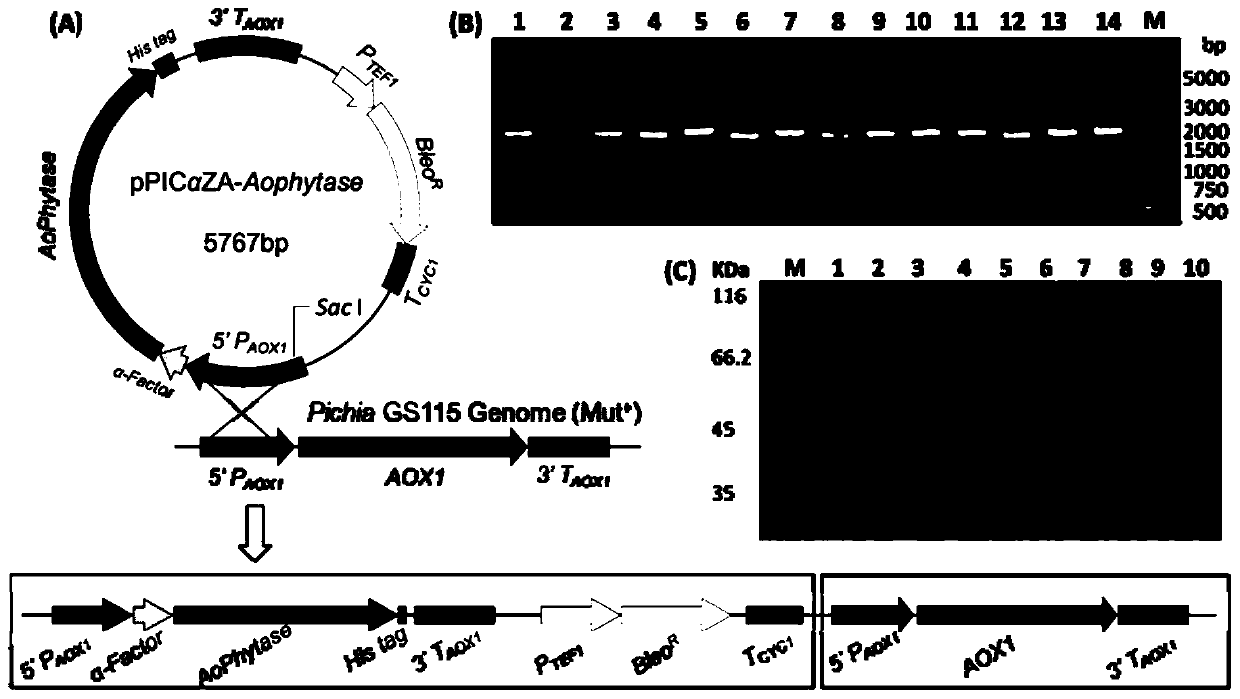
[0061] According to the AoPhytase amino acid sequence SEQ ID NO.1 and the nucleotide sequence SEQ ID NO.2 (GenbankaccessionNO.22896793), codon optimization was carried out for the Pichia pastoris expression system to obtain the AoPhytase gene sequence (SEQ ID in the sequence listing NO.3). Then use EcoRI and SalI to double digest the optimized gene fragment, connect it with the same double restriction vector pPICZαA at 16°C overnight, and transform the ligation product into competent DH5α cells, and put it on the LB solid medium plate containing Zeocin resistance Positive transformants were screened. Such as figure 1 As indicated, the expression vector pPICZαA-Aophytase was obtained.
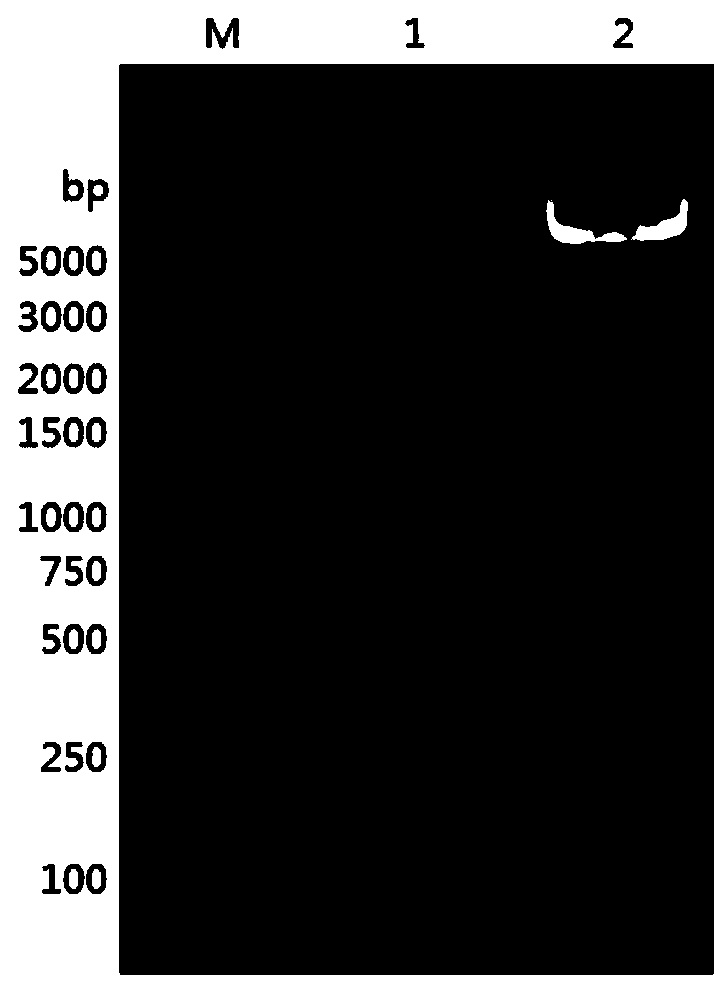
[0062] Take 20 μg of plasmid pPICZαA-Aophytase, add 2 μl of restriction enzyme Fast DigestSac Ⅰ enzyme and 50 μl of 10×FastDigest Buffer, add ddH2O to 500 μl, and react overnight at 37°C to make...
Embodiment 2
[0066] Embodiment 2: the purification of recombinant AoPhytase
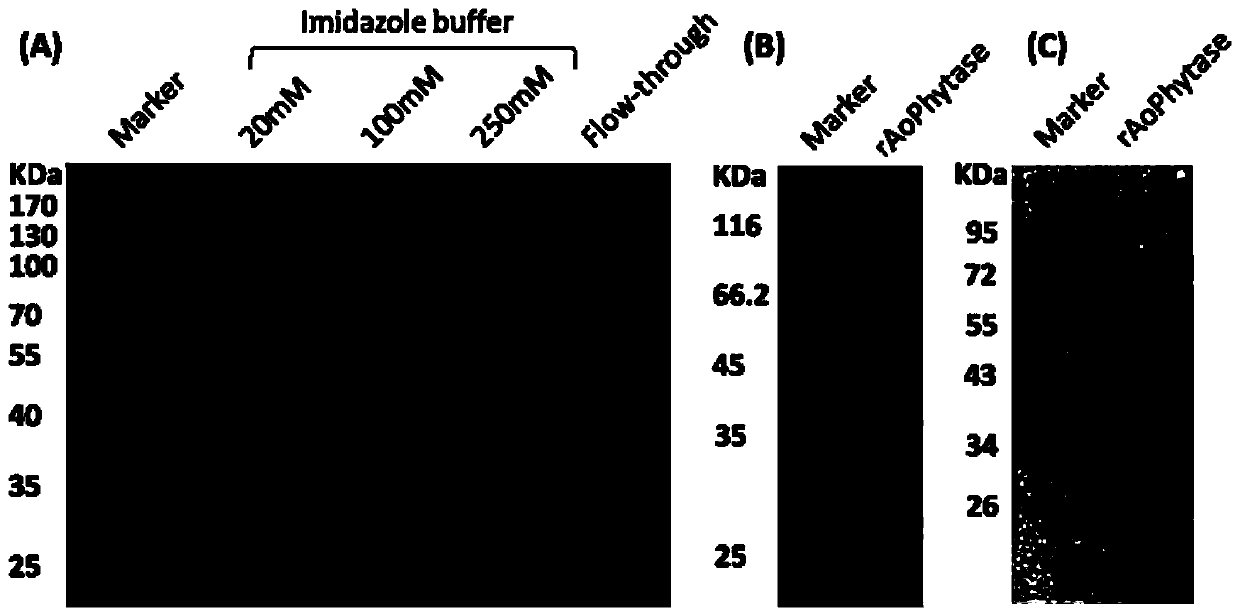
[0067] 1. Preparation of crude enzyme solution: Inoculate recombinant engineered Pichia pastoris into 50 mL of BMGY medium at a ratio of 1:1000, culture at 220 rpm at 30°C for about 48 hours, and measure OD600 = 4.6. Then the culture was centrifuged at 1500 rpm for 10 min, the supernatant was discarded, and the pellet was resuspended in 200 mL of BMMY medium, and the OD600 was measured to be 1.1, and induced at 220 rpm for 72 h at 30°C. The above culture solution was centrifuged at 4°C, 8000rpm for 10min, and the supernatant was the crude enzyme solution.
[0068] 2. Separation and purification of phytase: add 5mL Ni NTA Beads 6FF to the supernatant, and incubate at 4°C for 2h. Add the incubation product to the empty column tube and collect the effluent. The filler was washed with 20 mM imidazole buffer solution (10 mM Na2HPO4, 2 mM KH2PO4, 0.8% NaCl, 0.02% KCl, 5% Glycerol, 20 mM Imidazole, pH 6.0), and the wa...
Embodiment 3
[0069] Embodiment 3: Analysis of recombinant AoPhytase enzyme activity
[0070] 1. Drawing of phosphorus standard curve: measure 0, 2, 4, 6, 8 and 10ml of phosphorus standard stock solution in a 100ml volumetric flask, dilute to the mark, and make phosphorus content of 0, 0.1, 0.2, 0.3 , 0.4 and 0.5mmol / L standard curve determination liquid. Take six 15ml centrifuge tubes, add 1ml of the standard measurement solution of the above concentration, and then add 1ml of AMES chromogenic solution. After 20 minutes in a water bath at 50°C, cool to room temperature, measure the OD value at 700nm, and draw the OD-phosphorus concentration standard curves, such as Figure 4 shown.
[0071] 2. Determination of enzyme activity: After diluting the pure enzyme solution with a concentration of 0.088 mg / ml 10 times, take 100 μl of diluted enzyme solution and 900 μl of substrate solution (2mM sodium phytate dissolved in 100mM Tris-HCl buffer, pH 7.0) and mix , add 1ml of 10% trichloroacetic a...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com