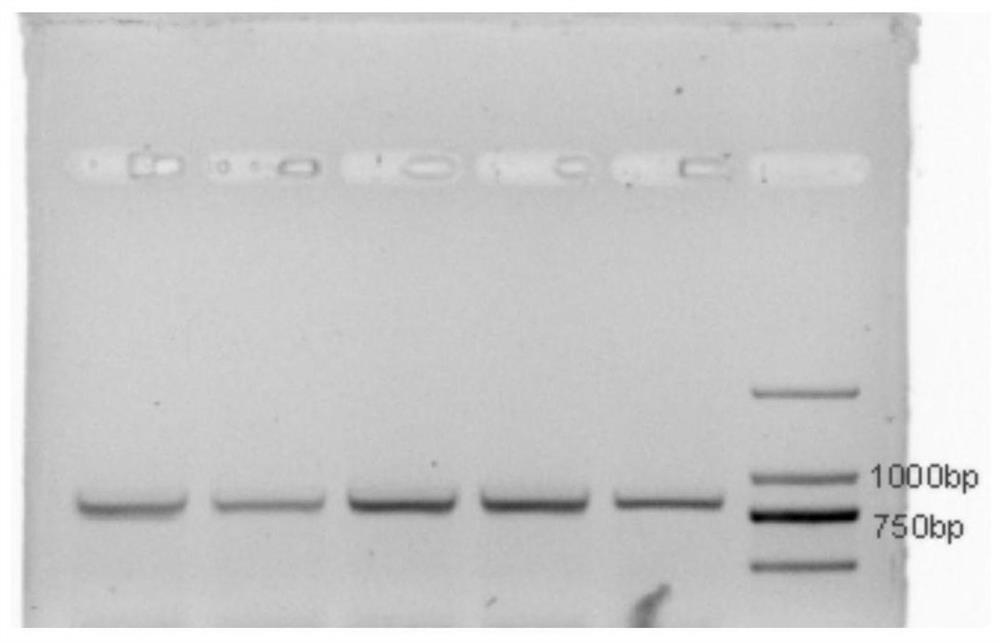
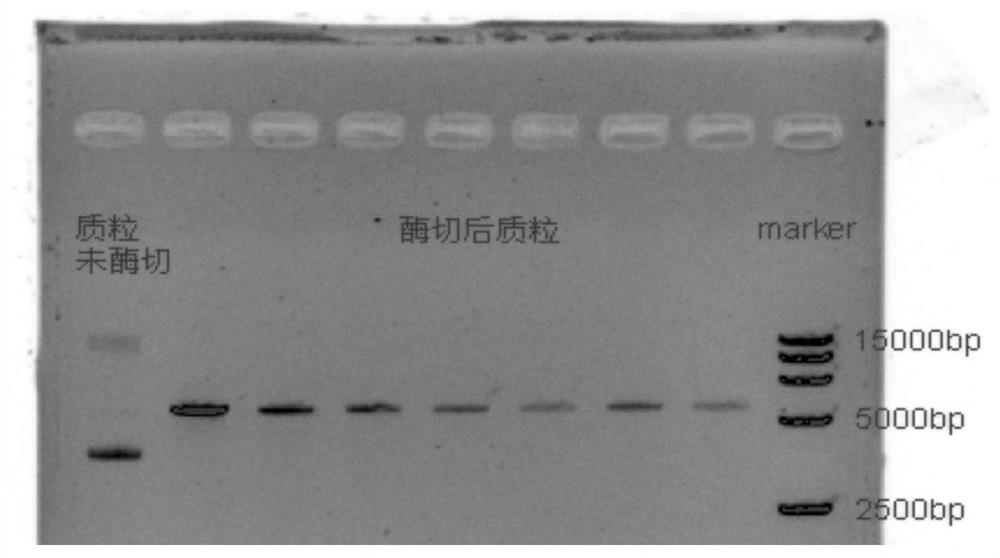
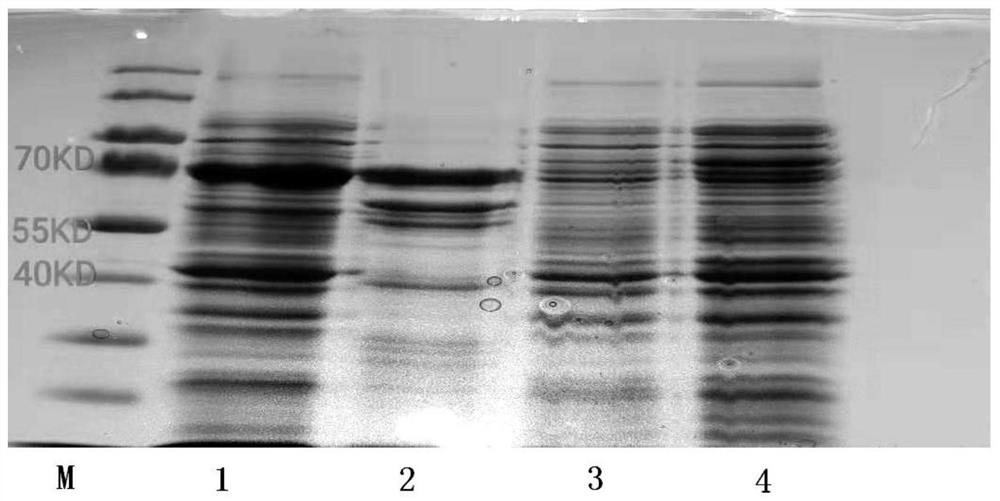
Method for Recombinant Expression and Purification of Vitellogenin in Channa sinensis
A technology of vitellogenin and Chinese snakehead, which is applied in the field of fusion protein expression and purification, can solve the problems of increasing separation and purification procedures, and the renaturation rate cannot reach 100%, achieving low cost, high recovery rate, and high expression level Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
specific Embodiment approach
[0054] The following embodiments will further illustrate the present invention in conjunction with the accompanying drawings, and the specific implementation methods are as follows:
[0055] The recombinant expression and purification of vitellogenin of Channa sinensis, equipped with the following experimental supplies: 1 μL recombinant PMAL-c5x vector, BL21 strain, 100 μg / mL ampicillin, 0.5 mM IPTG, PBS buffer, 1.0 MMTris-HCL, 300 mM NaCl, 0.5MEDTA, Amylmose affinity column, Factor Xa and QubitTMProtein Assay Kit protein concentration determination kit.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com