Preparation method of piperazine ethane sulfonic acid derivatives
A technology for piperazine ethanesulfonic acid and derivatives, which is applied in the field of preparation of piperazine ethanesulfonic acid derivatives, can solve the problems of difficult to obtain qualified products, difficult to industrialized large-scale production, low crude product content and the like, and achieves low inorganic salt content. , The effect of reducing production cost and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
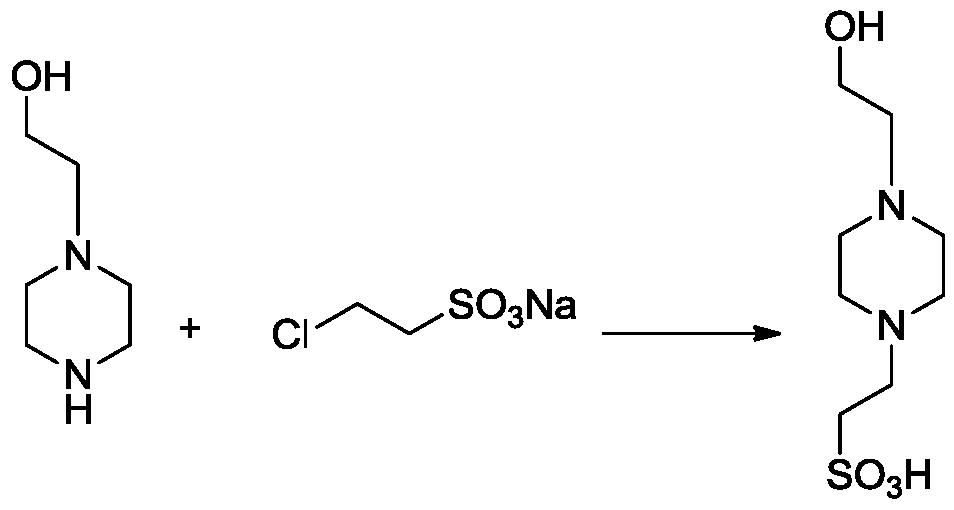
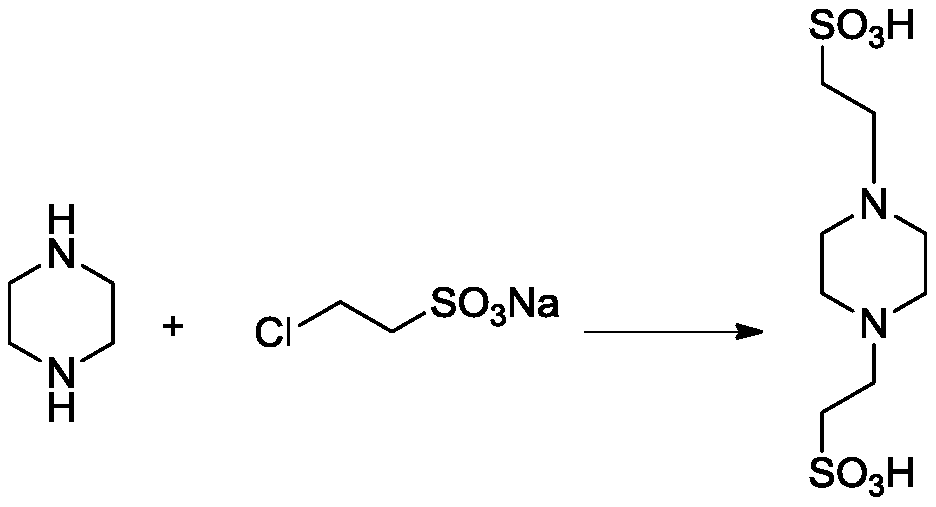
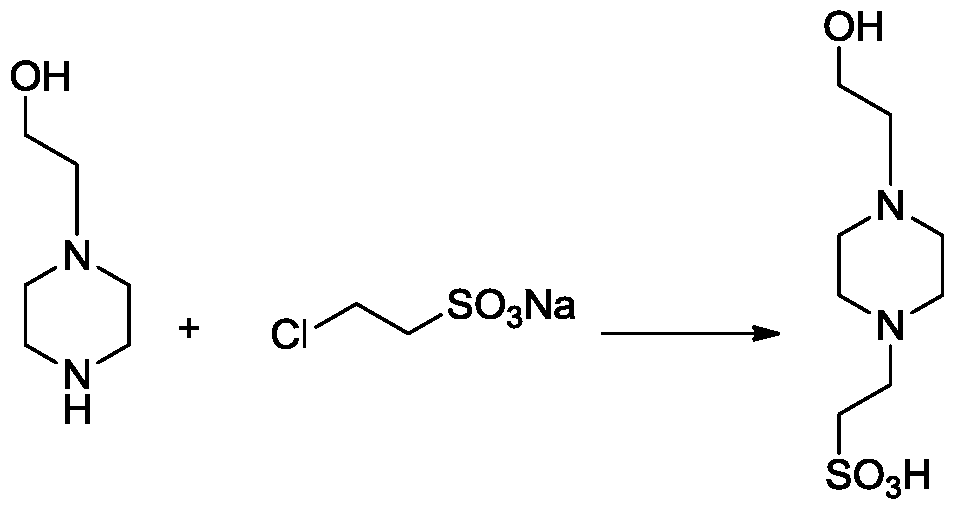
[0040] The invention provides a kind of preparation method of piperazineethanesulfonic acid derivative, comprises following equation:
[0041]
[0042] Include the following steps:
[0043] 1) Mix 1.2-2.2mol 2-hydroxyethylpiperazine, 1mol 2-chloroethylsulfonate sodium with 100-200mL water, stir evenly, raise the temperature to 70-90°C, stop heating until the system self-heats to 103 -105°C, heat to reflux for 20-60min;
[0044] 2) Cool the reaction solution to 50-70°C, add triethylamine to the reaction solution to adjust the pH to 7-8, boil with 1-2L absolute ethanol and 6-10g activated carbon, and filter while it is hot;
[0045] 3) Use glacial acetic acid to adjust the pH to 4-5, stir the filtrate, cool and crystallize, filter, and dry;
[0046] 4) Add 700-800mL of ethanol to dissolve the dried sample in step 3, heat to reflux, add 130-160mL of water, stir to dissolve, filter while it is hot, and cool and filter the filtrate to obtain 4-hydroxyethylpiperazineethanesulfo...
Embodiment 1
[0049] The preparation method of 4-hydroxyethylpiperazineethanesulfonic acid comprises the following steps:
[0050] 1) Mix 260g (2mol) 2-hydroxyethylpiperazine, 165g (0.94mol) sodium 2-chloroethylsulfonate with 120mL water, stir evenly, the reaction solution becomes clear, the pH is about 10, and the temperature is raised to 85°C , stop heating until the system self-heats to 103-105 ° C, heat and reflux for 30 minutes, the reaction is over, and the pH is about 8;
[0051] 2) Cool the reaction solution to 60°C, add triethylamine to the reaction solution to adjust the pH to 7-8, boil with 1.5L of absolute ethanol and 8g of activated carbon for 30min, and filter while hot;
[0052] 3) Use glacial acetic acid to adjust the pH of the filtrate to 5, stir the filtrate, cool to 5° C. to crystallize overnight, filter, and dry to obtain 310 g of crude product, which is tested by titration after drying, and the content is 95%;
[0053] 4) Add 750mL of ethanol to the sample obtained in ...
Embodiment 2
[0056] The preparation method of 4-hydroxyethylpiperazineethanesulfonic acid comprises the following steps:
[0057] 1) Mix 244.4g (1.88mol) of 2-hydroxyethylpiperazine, 165g (0.94mol) of sodium 2-chloroethylsulfonate with 125mL of water, stir evenly, the reaction solution becomes clear, the pH is about 10, and the temperature is raised to 80°C, stop heating until the system self-heats to 103-105°C, heat and reflux for 30 minutes, the reaction is over, and the pH is about 8;
[0058] 2) Cool the reaction solution to 60°C, add triethylamine to the reaction solution to adjust the pH to 7-8, boil with 1.6L absolute ethanol and 8g activated carbon for 30min, and filter while hot;
[0059] 3) Use glacial acetic acid to adjust the pH of the filtrate to 5, stir the filtrate, cool to 5° C. to crystallize overnight, filter, and dry; 327 g of crude product is obtained, and the content is 95% after drying and titration test;
[0060] 4) Add 800mL of ethanol to the dried sample in step 3...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com