Digital PCR reagent kit and detection method for quantitative detection of EB virus nucleic acid
A technology for quantitative detection of Epstein-Barr virus, applied in the direction of microorganism-based methods, biochemical equipment and methods, and microorganism measurement/inspection, can solve inaccurate detection results, uncertain detection results whether the virus is positive, sensitivity and Poor specificity and other issues, to achieve high tolerance, avoid deviation, and easy standardization
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
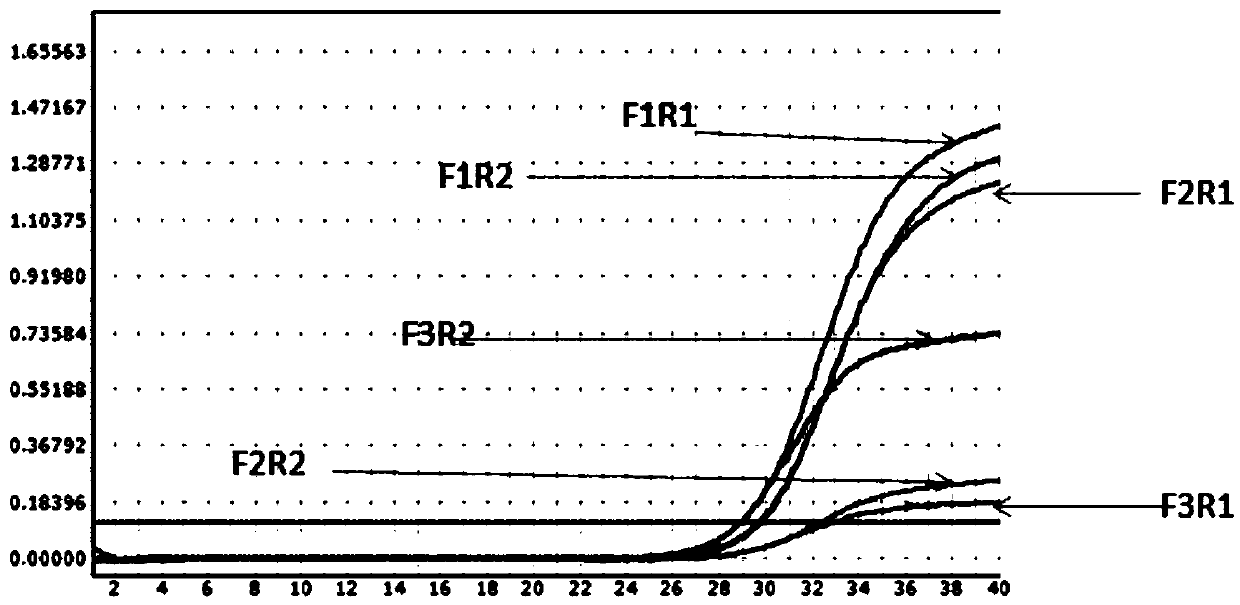
[0069] Example 1: Primer Screening
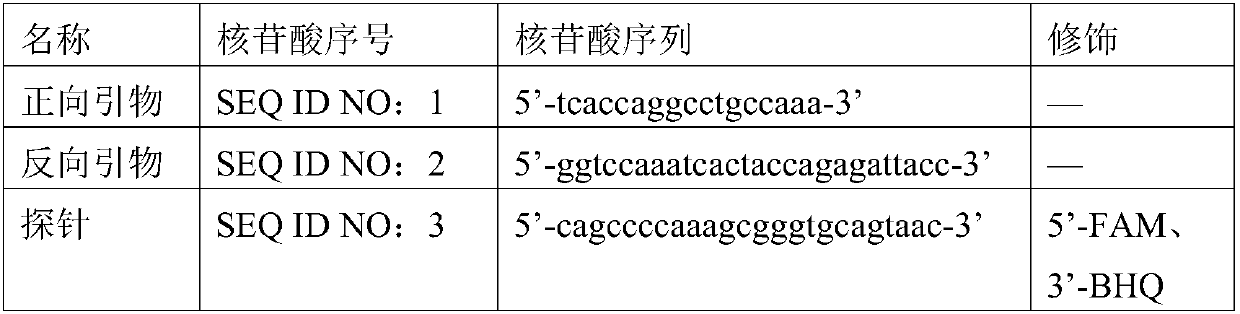
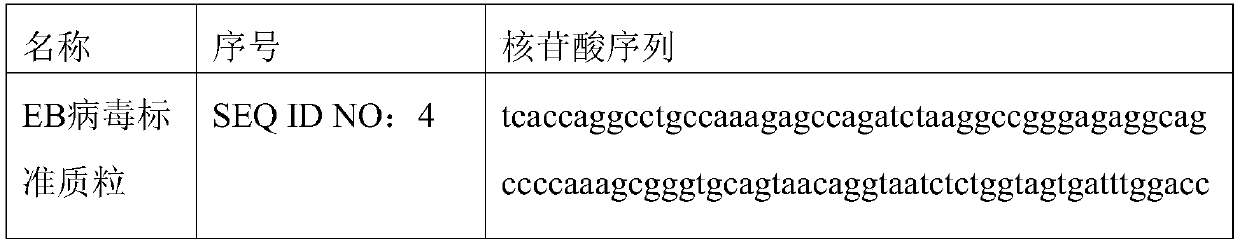
[0070] 1. Design of primers and probes: refer to relevant domestic and foreign literature, select EBV-specific conserved sequences as target detection fragments, and pass through the National Center for Biotechnology Information (NCBI, http: / / www.ncbi.nlm.nih.gov), The specific conserved sequence of Epstein-Barr virus was obtained, and the professional design software BeaconDesigner 7.0 was used to design specific primers and probes suitable for ddPCR.
[0071] This example gives the process of screening the best primers, and selects several pairs of alternative primers designed by software for screening, and the alternative primers are shown in Table 5.
[0072] table 5
[0073]
[0074] 2. Screening of primers
[0075] 2.1 The primers are randomly matched into six pairs: F1R1, F1R2, F2R1, F2R2, F3R1, F3R2;
[0076] Specific probe P: the fluorescent reporter gene at the 5' end is FAM, and the quencher group at the 3' end is BHQ.
[...
Embodiment 2
[0081] Embodiment 2: kit composition
[0082] The specific composition of the kit is shown in Table 6:
[0083] Table 6
[0084]
Embodiment 3
[0085] Embodiment 3: Specificity test
[0086] 1. Materials: serum samples from patients infected with hepatitis B virus, human cytomegalovirus, and Epstein-Barr virus and healthy people.
[0087] 2. Method: Use a commercial extraction kit to extract the serum samples of patients infected with hepatitis B virus, human cytomegalovirus, and Epstein-Barr virus, and the serum samples of healthy people to obtain DNA templates, and use the kit of the embodiment of the present invention to obtain DNA templates. Perform ddPCR amplification detection to observe whether the kit has non-specific reactions.
[0088] 3. Results: DNA from serum samples from patients infected with hepatitis B virus, human cytomegalovirus, and Epstein-Barr virus and healthy people was amplified by a PCR instrument, detected by a droplet reader, and automatically read by QuantaSoft software. Take and analyze. The specific experimental results are shown in Table 7.
[0089] Table 7
[0090] name ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com