Preparation method of tubular g-C<3>N<4> with visible-light response function
A technology of g-c3n4 and visible light, applied in the field of preparation of tubular g-C3N4, can solve the problems of increased cost, time-consuming, cumbersome experimental process, etc., and achieve the effects of reduced energy consumption, simple process and low cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0021] Visible light-responsive tubular g-C 3 N 4 The preparation method comprises the following steps:
[0022] 1) Melamine and cyanuric acid are mixed and dissolved in ultrapure water with a mass ratio of 1:0.01-1, and magnetically stirred for 30-90 minutes to form a uniform mixed solution;
[0023] 2) Then, transfer the above solution to a stainless steel autoclave, and heat at 160-200°C for 12-24 hours to obtain the precursor of the synthetic product;
[0024] 3) washing the obtained precursor with ultrapure water three times, and drying at 50-100° C. for 8-12 hours;
[0025] 4) Then calcinate the dried precursor in nitrogen; at 2-5°C min -1 The heating rate is maintained at 550 ° C for 3-8 hours, and finally a tubular g-C 3 N 4 catalyst of light.
[0026] Alternatively, to prepare block g-C 3 N 4 Photocatalyst as a control: First, put 1-10g of melamine into an alumina crucible with a lid, and heat it in a muffle furnace at 550°C for 2-5°C min -1 The heating rate ...
Embodiment 1
[0032] 1) Preparation of block g-C 3 N 4 catalyst of light.
[0033] First, 3 g of melamine was put into an alumina crucible with a lid, and heated at 550 °C in a muffle furnace at 2.3 °C min -1 Calcined at a heating rate of 4 hours.
[0034] 2) Preparation of tubular g-C 3 N 4 catalyst of light
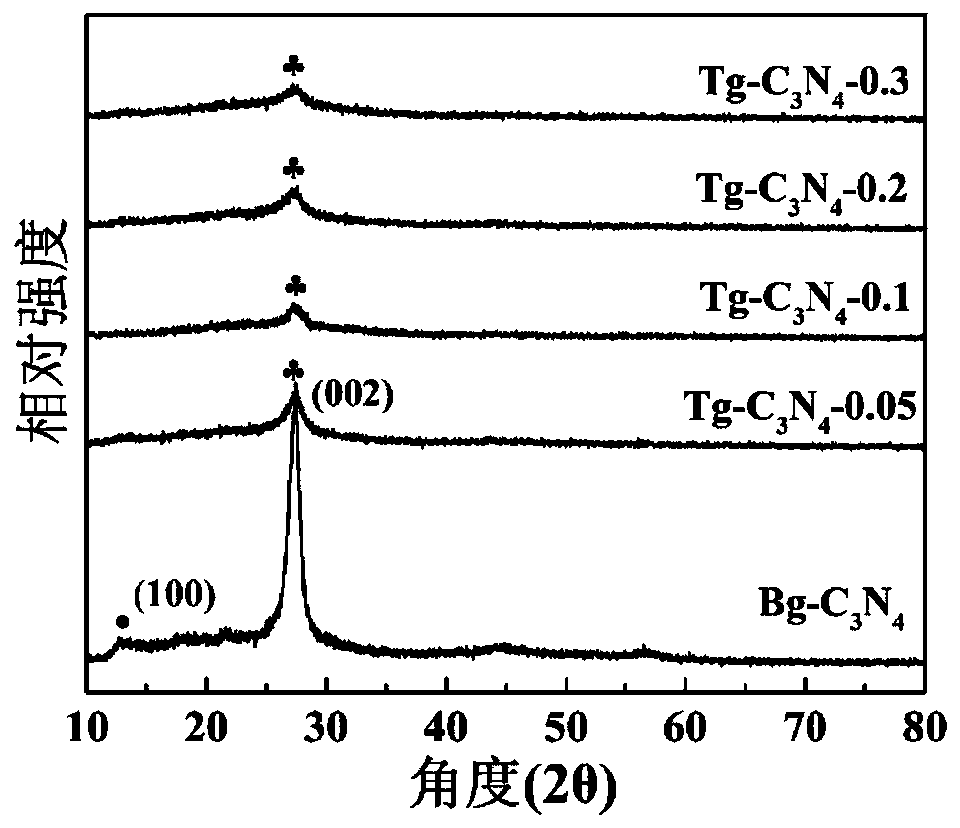
[0035] Dissolve 1 g of melamine and different amounts of cyanuric acid (0.05, 0.1, 0.2, 0.3) in 60 ml of deionized water, stir magnetically for 60 minutes to form a homogeneous solution; then, transfer the above mixed solution to 100 ml of Teflon stainless steel high pressure heated at 180°C for 24 hours in an autoclave; after that, the obtained intermediate sample was washed three times with ultrapure water and dried at 80°C for 12 hours. The resulting intermediate sample was calcined in nitrogen; at 2.3°C min -1 The rate was maintained at 550 °C for 4 hours.
[0036] 3) Photocatalyst visible light hydrogen production
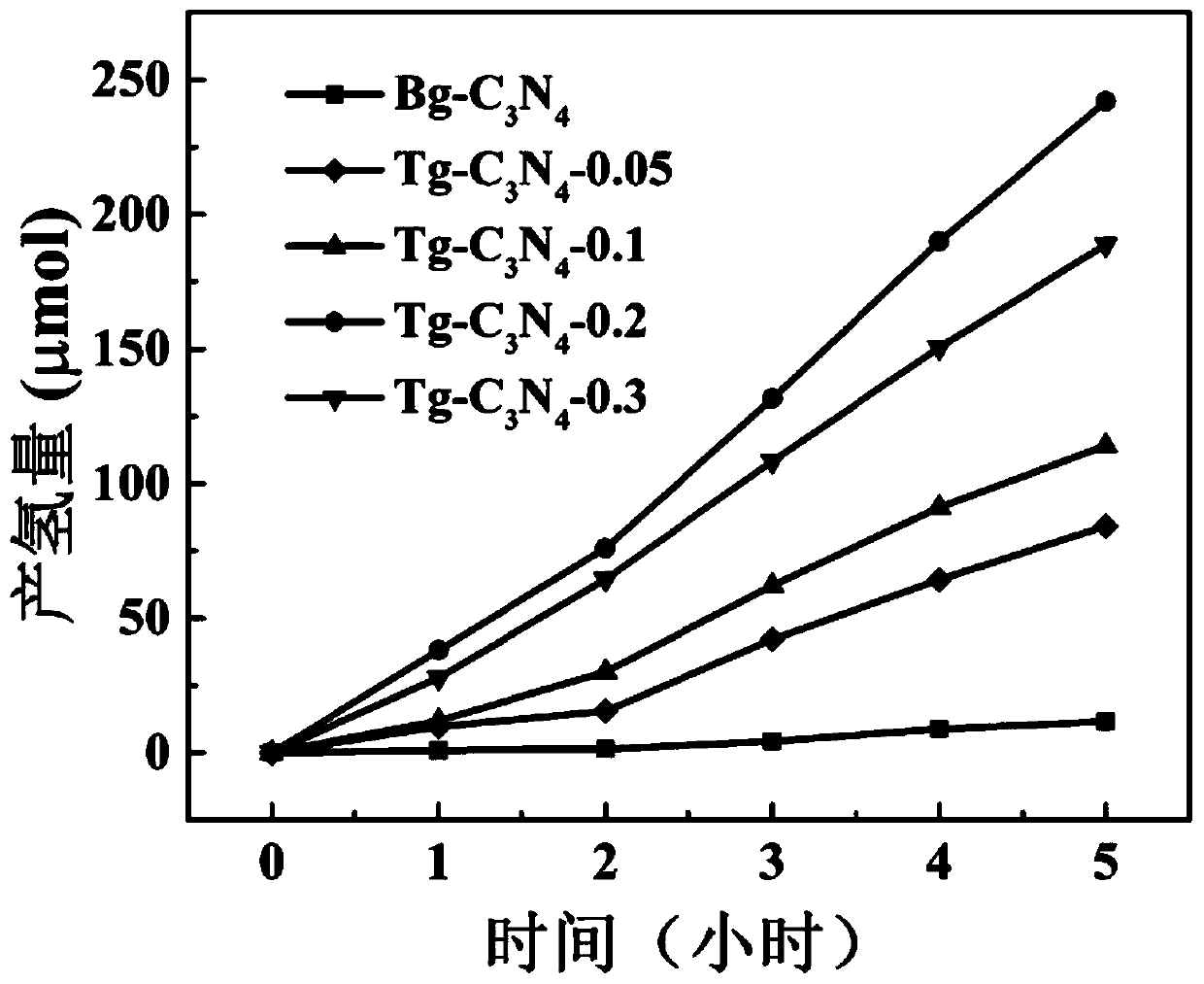
[0037] Disperse 0.05g sample evenly in 100ml 20vol% tr...
Embodiment 2
[0039] Preparation of tubular g-C 3 N 4 catalyst of light
[0040] Dissolve 1 g of melamine and different amounts of cyanuric acid (0.05, 0.1, 0.2, 0.3) in 60 ml of deionized water, stir magnetically for 60 minutes to form a homogeneous solution; then, transfer the above mixed solution to 100 ml of Teflon stainless steel high pressure In the still, heated at 160°C for 24 hours; after that, the obtained intermediate sample was washed three times with ultrapure water, and dried at 80°C for 12 hours. The resulting intermediate sample was calcined in nitrogen; at 2.3°C min -1 The rate was maintained at 550 °C for 4 hours.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com