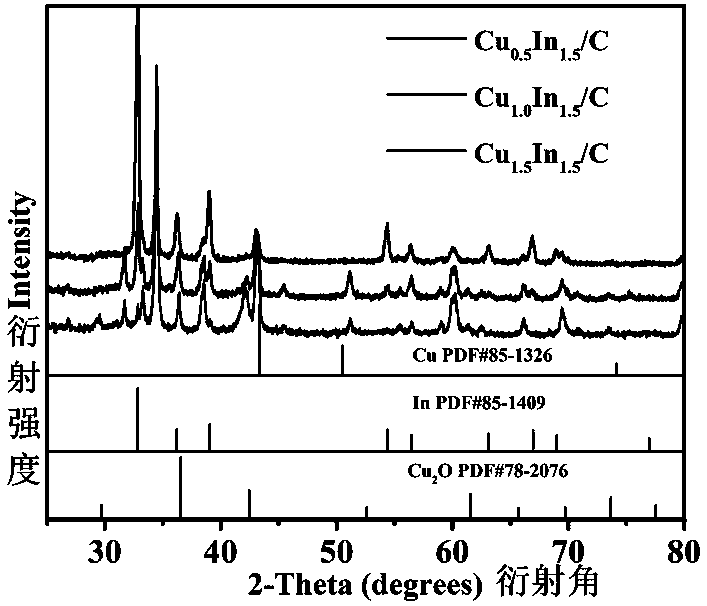
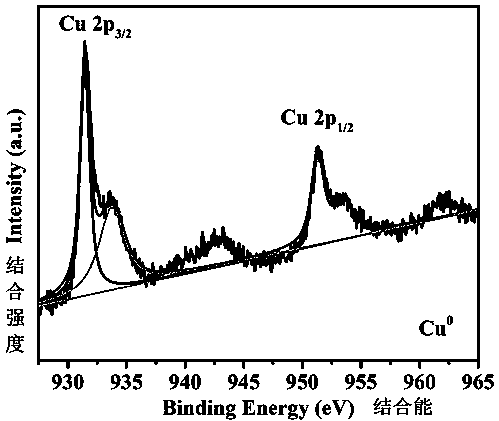
Method for synthesizing copper-indium/carbon bimetal nano material through one-step reduction method and application of copper-indium/carbon bimetal nano material
A bimetallic nano-reduction technology, applied in the field of electrochemistry, can solve the problems of copper-indium/carbon bimetallic nanomaterial electrodes that have not yet been discovered, achieve excellent electrochemical activity and stability, easy industrial implementation, and simple operation process easy effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] The one-step reduction method synthesizes copper-indium / carbon bimetallic nanomaterial electrodes, including the following steps:
[0031] Step 1: Dissolve 1.5 mmol indium chloride in 20 mL, 0.1 M hydrochloric acid solution to form solution A;
[0032] Step 2: prepare 0.2 M citric acid solution with deionized water, i.e. solution B;
[0033] Step 3: Pour 20mL of solution A into 20mL of solution B under magnetic stirring, then add 212.8 mg of carbon black to form a suspension C, and ultrasonicate for 30 minutes until uniformly dispersed;
[0034] Step 4: Vigorously stir the uniformly dispersed suspension C in an ice bath, slowly drop into the newly prepared 25 mL, 0.6 M sodium borohydride solution to form a suspension D, and then stir at 200 rpm for 2 h;
[0035] Step 5: Centrifuge the suspension D at 10,000 rpm, wash the product with deionized water several times, and dry it under vacuum at 60 °C for 12 h to obtain the catalyst In 1.5 / C.
Embodiment 2
[0037] The one-step reduction method synthesizes copper-indium / carbon bimetallic nanomaterial electrodes, including the following steps:
[0038] Step 1: Dissolve 1.0 mmol copper chloride in 20 mL, 0.1 M hydrochloric acid solution to form solution A;
[0039] Step 2: prepare 0.2 M citric acid solution with deionized water, i.e. solution B;
[0040] Step 3: Pour 20mL of solution A into 20mL of solution B under magnetic stirring, then add 212.8 mg of carbon black to form a suspension C, and ultrasonicate for 30 minutes until uniformly dispersed;
[0041] Step 4: Vigorously stir the uniformly dispersed suspension C in an ice bath, slowly drop into the newly prepared 25 mL, 0.4 M sodium borohydride solution to form a suspension D, and then stir at 200 rpm for 2 hours;
[0042] Step 5: Centrifuge the suspension D at 10,000 rpm, wash the product with deionized water several times, and dry it in vacuum at 60 °C for 12 h to obtain the catalyst Cu 1.0 / C.
Embodiment 3
[0044] The one-step reduction method synthesizes copper-indium / carbon bimetallic nanomaterial electrodes, including the following steps:
[0045] Step 1: Dissolve 0.5 mmol indium chloride and 0.5 mmol copper chloride in 20 mL, 0.1 M hydrochloric acid solution to form solution A;
[0046] Step 2: prepare 0.2 M citric acid solution with deionized water, i.e. solution B;
[0047] Step 3: Pour 20mL of solution A into 20mL of solution B under magnetic stirring, then add 212.8 mg of carbon black to form a suspension C, and ultrasonicate for 30 minutes until uniformly dispersed;
[0048] Step 4: Vigorously stir the uniformly dispersed suspension C in an ice bath, slowly drop into the newly prepared 25 mL, 0.4 M sodium borohydride solution to form a suspension D, and then stir at 200 rpm for 2 hours;
[0049]Step 5: Centrifuge the suspension D at 10,000 rpm, wash the product with deionized water several times, and dry it in vacuum at 60 °C for 12 h to obtain the catalyst Cu 0.5 In ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com