A kind of perfluoropolyether imidazoline compound and its preparation method and application
A technology of perfluoropolyether imidazoline and perfluoropolyether carboxylic acid is applied in the field of perfluoropolyether imidazoline compounds and their preparation, which can solve the problems of refractory degradation, harmful bioaccumulation, biological and human harm, etc. Low environmental pollution, high yield and strong adsorption
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
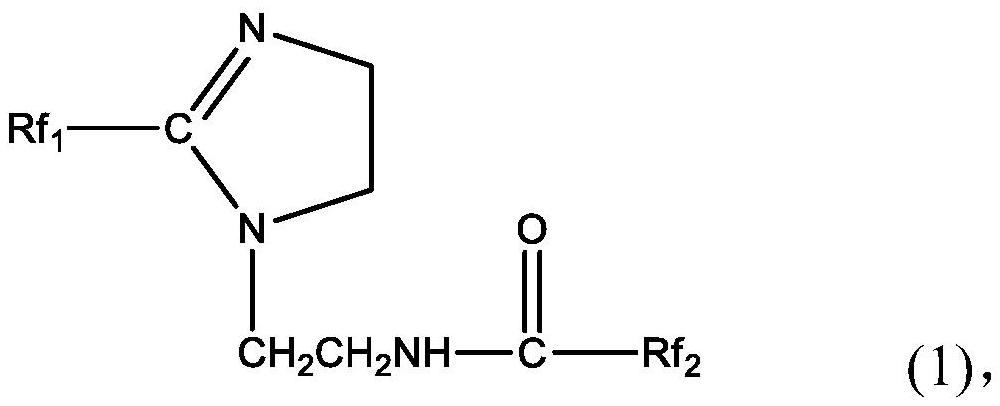
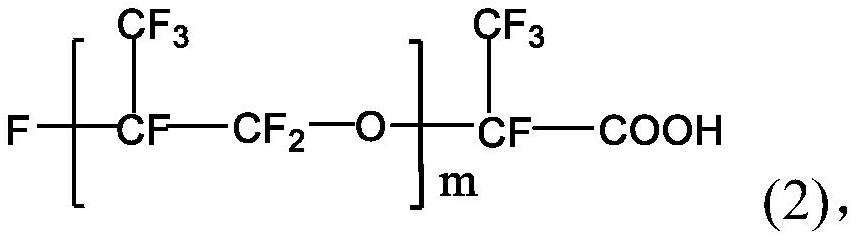
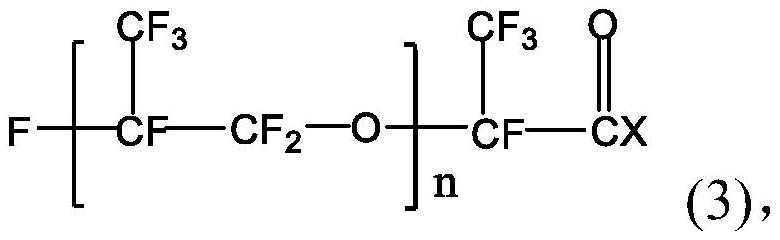
[0030] The perfluoropolyether imidazoline compound is prepared from perfluoropolyether carboxylic acid, diethylenetriamine and perfluoropolyether acid halide. The specific preparation method may include: firstly mix perfluoropolyether carboxylic acid, diethylenetriamine and water-carrying agent, stir and raise the temperature to 140-160°C, and maintain the reaction temperature for 0.5-5h to obtain the first intermediate; then continue Stir and heat up to 180-210°C, and maintain the reaction temperature for 0.5-5 hours; then cool and purify to obtain the second intermediate; continue to mix the obtained second intermediate with the acid-binding agent, and add dropwise in a protective gas atmosphere After the perfluoropolyether acid halide is added dropwise, the temperature is raised to 40-80°C, and the reaction temperature is maintained for 20-30 hours to obtain the perfluoropolyether imidazoline compound.
[0031] The structure of the perfluoropolyether carboxylic acid used in...
Embodiment 1
[0051] Mix 50g of perfluoropolyether carboxylic acid (994g / mol, m=5), 6.23g of diethylenetriamine, and 11g of xylene, stir and raise the temperature to 160°C, and maintain this temperature for 2.5h to obtain the first intermediate; then The temperature of the reaction was further raised to 200° C., and the reaction temperature was maintained for 2.5 h. After cooling, 51.28 g of the second intermediate was obtained by distillation and purification under reduced pressure, with a yield of 96.08%.
[0052] Mix 50g of the second intermediate and 3ml of triethylamine, and add 50g of perfluoropolyetheryl fluoride (996g / mol, n=5) dropwise under anhydrous and oxygen-free nitrogen atmosphere. After the dropwise addition, the temperature is raised to 55°C, After reacting for 25 hours, 92.80 g of perfluoropolyether imidazoline compound was obtained, with a yield of 96.68%. The perfluoropolyether imidazoline compound obtained here is designated as corrosion inhibitor A.
Embodiment 2
[0054] Mix 100g of perfluoropolyether carboxylic acid (828g / mol, m=4), 13.71g of diethylenetriamine, and 40g of xylene, stir and raise the temperature to 155°C, and maintain this temperature for 2h to obtain the first intermediate; then The temperature of the reaction was continued to rise to 190° C., and the reaction temperature was maintained for 2 h. After cooling, 104.56 g of the second intermediate was obtained by distillation and purification under reduced pressure, with a yield of 96.73%.
[0055] Mix 100g of the second intermediate and 3ml of triethylamine, and add 125g of perfluoropolyetheryl fluoride (996g / mol, n=5) dropwise under an anhydrous and oxygen-free nitrogen atmosphere. After reacting for 24 hours, 195.45 g of perfluoropolyether imidazoline compound was obtained, with a yield of 93.49%. The perfluoropolyether imidazoline compound obtained here is designated as corrosion inhibitor B.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com