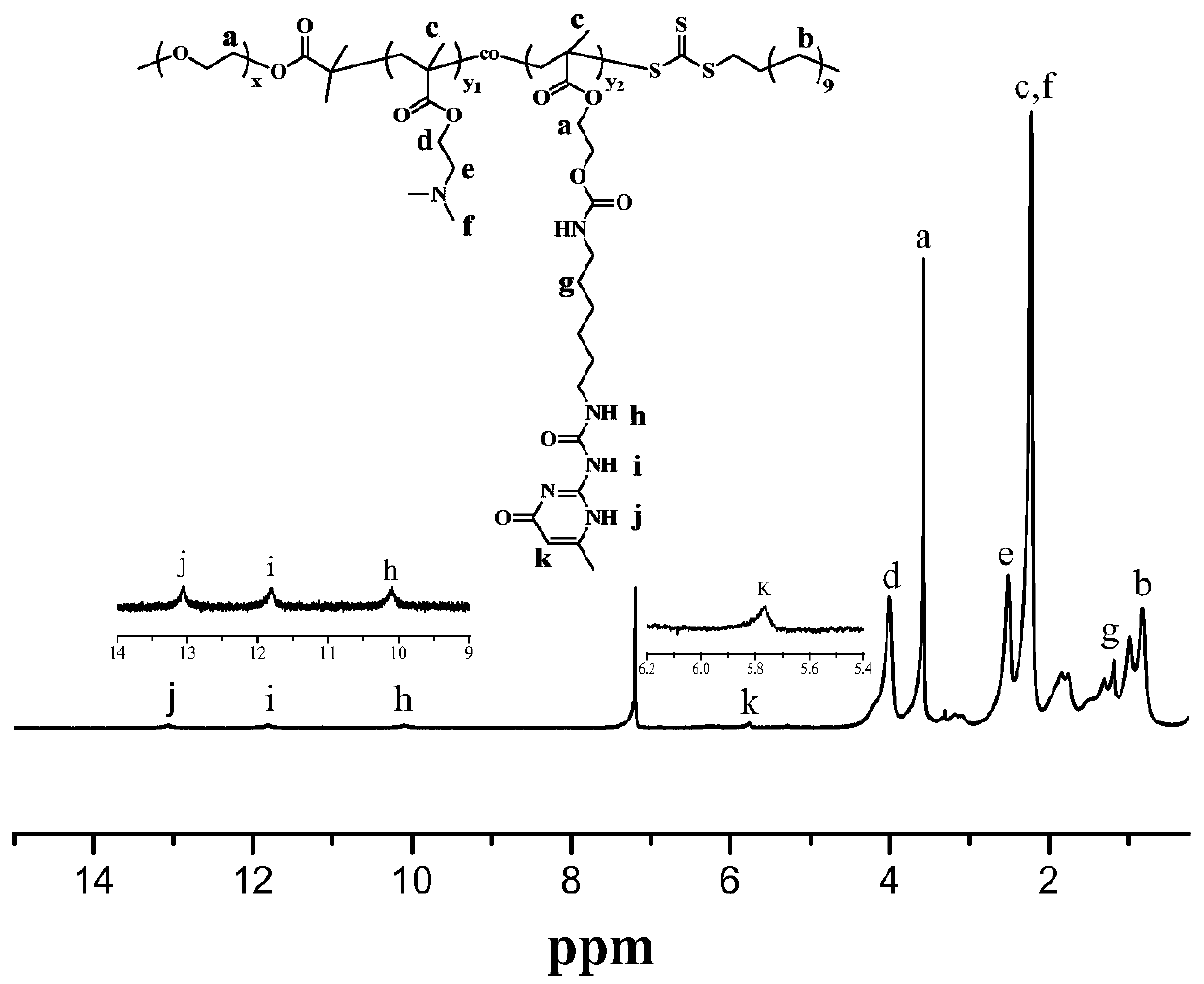
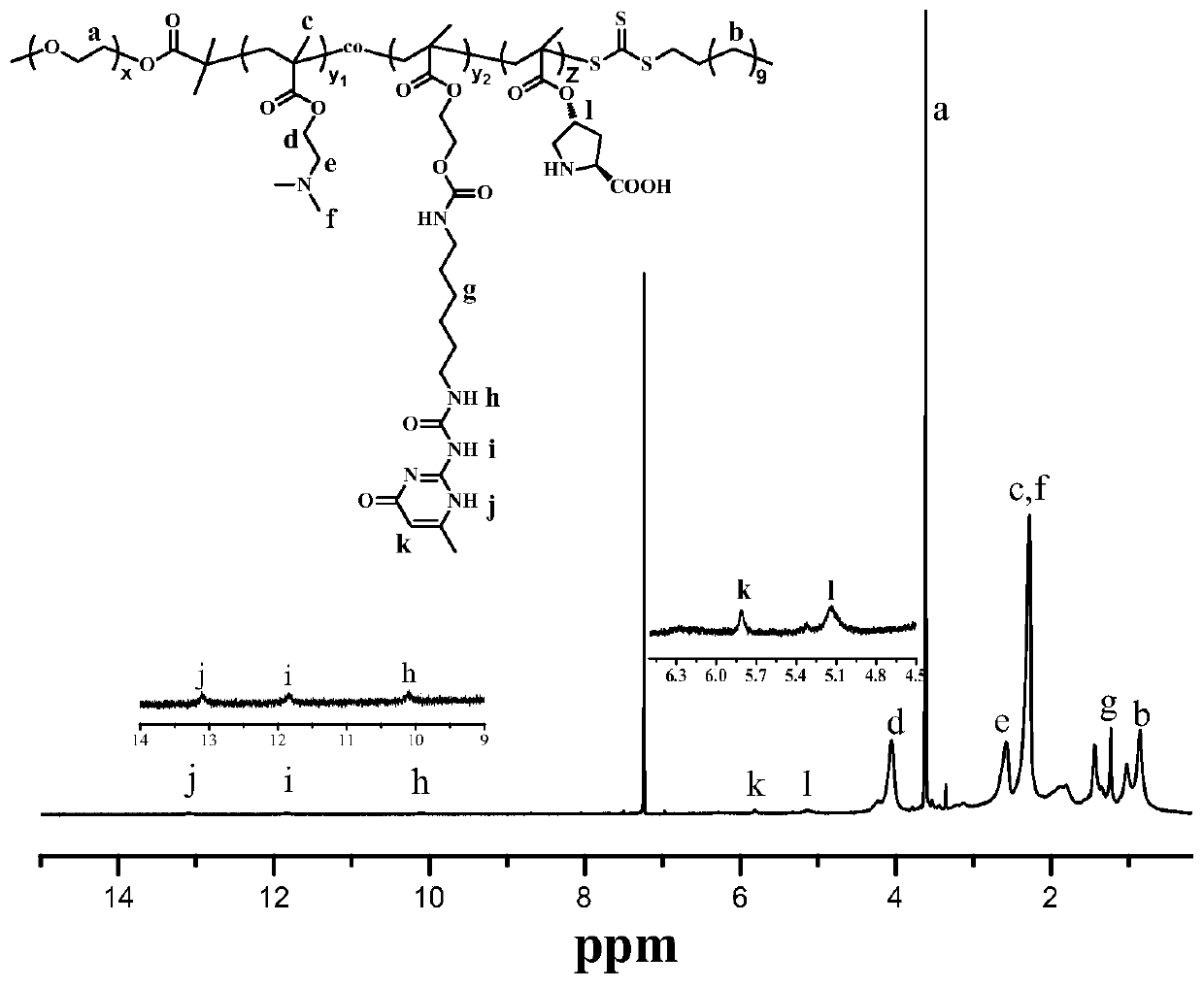
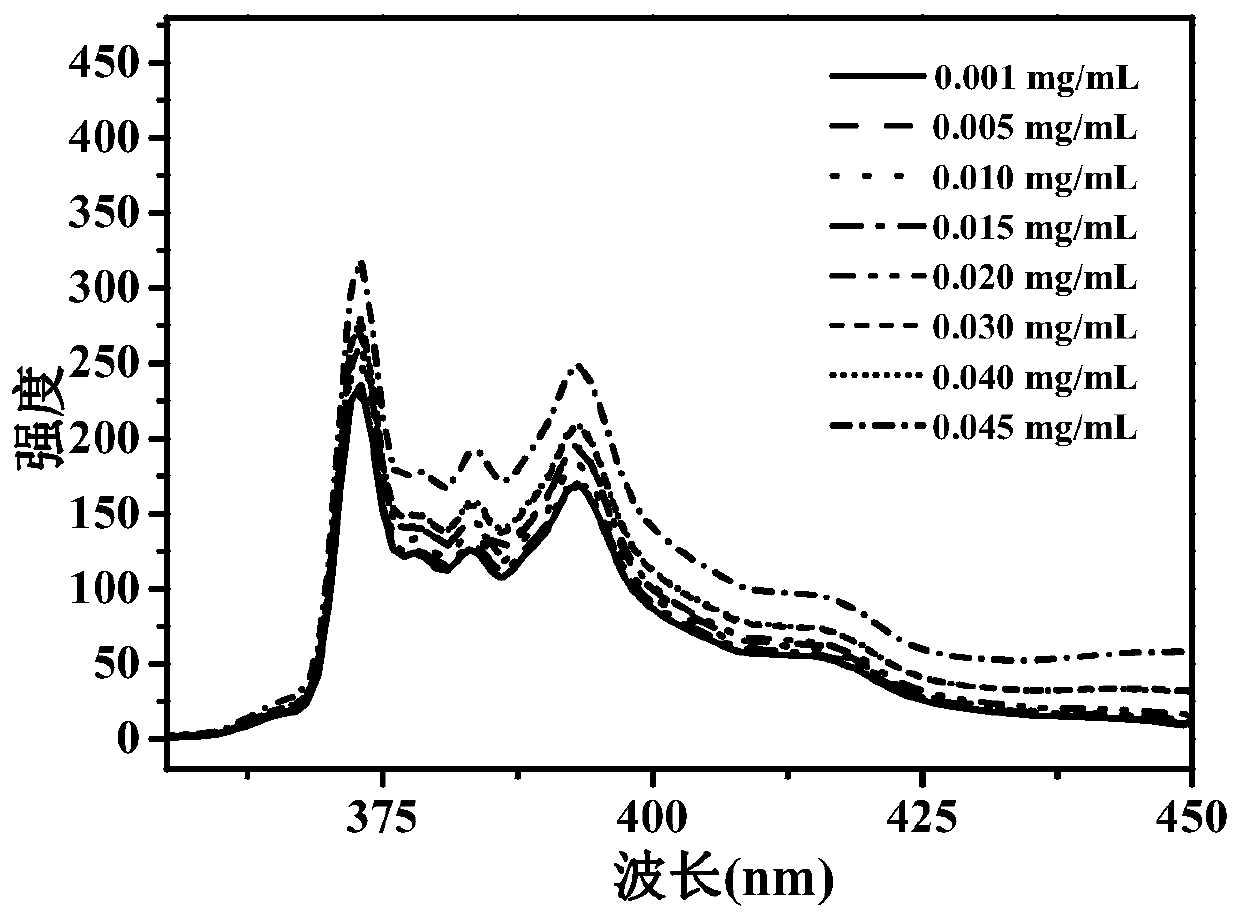
Water-soluble copolymer immobilized L-proline catalyst and preparation method and application thereof
A technology of water-soluble copolymers and proline, which is applied in the direction of physical/chemical process catalysts, catalytic reactions, organic chemical methods, etc., can solve the problems of improved cycle performance, reduced catalytic activity and selectivity, and achieve excellent cycle Use performance, good catalytic performance, the effect of simple method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0044]
[0045] 1. Dissolve CTA (6.552g, 18mmol), dry mPEG (4.0000g, 2mmol) and DMAP (0.2440g, 2mmol) in 60mL CH 2 Cl 2 After being completely dissolved, EDC (1.5336g, 8mmol) was added, reacted at 25°C for three days, and the insoluble matter was removed. The solution was concentrated and precipitated twice in ether, and the obtained solid was dissolved in 80mL CH 2 Cl 2 In, with saturated Na 2 CO 3 The aqueous solution was washed three times, and the obtained organic phase was dried over anhydrous sodium sulfate for 12 hours. Filtration, after rotary evaporation removes most of solvent, precipitate in excess glacial ether, obtain light yellow powder solid, the macromolecular chain transfer agent (mPEG) shown in formula I-1 33 -CTA).
[0046] 2. The mPEG 33 - CTA (0.4668g, 0.2mmol), DMAEMA (1.4444g, 9.2mmol), UPy-HEMA (0.3176g, 0.8mmol) and AIBN (0.0164g, 0.1mmol) were added to a 50mL Shrek tube, and 5mL DMSO was added Make it dissolve, freeze-thaw and degas three ti...
Embodiment 2
[0052]
[0053] 1. Dissolve CTA (7.2800g, 20mmol), dry mPEG (10.0000g, 5mmol) and DMAP (0.2440g, 2mmol) in 60mL CH 2 Cl 2 After being completely dissolved, EDC (1.9170g, 10mmol) was added, reacted at 25°C for three days, and the insoluble matter was removed. The solution was concentrated and precipitated twice in ether, and the obtained solid was dissolved in 80mL CH 2 Cl 2 In, with saturated Na 2 CO 3 The aqueous solution was washed three times, and the obtained organic phase was dried over anhydrous sodium sulfate for 12 hours. Filtration, after rotary evaporation removes most of solvent, precipitate in excess glacial ether, obtain light yellow powder solid, the macromolecular chain transfer agent (mPEG) shown in formula I-2 39 -CTA).
[0054] 2. The mPEG 39 -CTA (0.7002g, 0.3mmol), DMAEMA (1.5700g, 10mmol), UPy-HEMA (0.4367g, 1.1mmol) and AIBN (0.0164g, 0.1mmol) were added to a 50mL Shrek tube, and 5mL DMSO was added to make it Dissolve, freeze-thaw and degas thre...
Embodiment 3
[0058]
[0059] 1. Dissolve CTA (8.008g, 22mmol), dry mPEG (6.0000g, 3mmol) and DMAP (0.2440g, 2mmol) in 60mL CH 2 Cl 2 After being completely dissolved, EDC (2.3004g, 12mmol) was added, reacted at 25°C for three days, and the insoluble matter was removed. The solution was concentrated and precipitated twice in diethyl ether, and the obtained solid was dissolved in 80mL CH 2 Cl 2 In, with saturated Na 2 CO 3 The aqueous solution was washed three times, and the obtained organic phase was dried over anhydrous sodium sulfate for 12 hours. Filtration, after rotary evaporation removes most of solvent, precipitate in excess glacial ether, obtain light yellow powder solid, the macromolecular chain transfer agent (mPEG) shown in formula I-3 35 -CTA).
[0060] 2. The mPEG 35 -CTA (0.2334g, 0.1mmol), DMAEMA (1.4915g, 9.5mmol), UPy-HEMA (0.1985g, 0.5mmol) and AIBN (0.0164g, 0.1mmol) were added to a 50mL Shrek tube, and 5mL DMSO was added to make It was dissolved, degassed three...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com