Hybridoma cell strain 6F9, antibody and application of hybridoma cell strain 6F9
A hybridoma cell line, monoclonal antibody technology, applied in application, antibody, anti-receptor/cell surface antigen/cell surface determinant immunoglobulin, etc., to achieve the effect of good specificity and high affinity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] Example 1: Construction of CHO-K1 stable cell lines overexpressing human CD70 and cynomolgus monkey CD70
[0027] 1) Shuttle carrier construction
[0028] Human CD70 protein sequence
[0029] NP_001243 CD70 antigen isoform 1[Homo sapiens]
[0030] MPEEGSGCSVRRRPYGCVLRAALVPLVAGLVICLVVCIQRFAQAQQQLPL ESLGWDVAELQLNHTGPQQDPRLYWQGGPALGRSFLHGPELDKGQLRIHRDGIYMVHIQVTLAICSSTTASRHHPTTLAVGICSPASRSISLLRLSFHQGCTIASQRLTCTNLTGTLLPSRNTDETFFGVQWVRP
[0031] 21,118Da, 193aa, 1-17 is the intracellular domain, 18-38 is the transmembrane helix, 39-193 is the extracellular domain.
[0032] Cynomolgus monkey CD70 protein sequence
[0033] XP_005587773PREDICTED:CD70 antigen[Macaca fascicularis]
[0034] MNGPRKNEVEREIGRSGGEGLGTGNSVAHPRPLPGPSGNHLHPLCELQTGSSWREFPLANRSSPSPRPAGHPQRGAGWSPDKLRQVDAQEPREGAAVAFLPFPAALCAPLAPPALAEVIAAAMPEEGSGCAVRRRPYACVLRAAVVPLVAGLAICLVVCVQRLSRAQQQLPLESLGWDIAELQLNHTGPQQDPRLYWQGGPALGRSFLRGPELDKGQLRIRRDGIYMVHIQVTLAICSSTSTSRHHHPTTLAVGICSPASRSISLLRLSFHQGCTIASQRLTPLARGDTLCT...
Embodiment 2
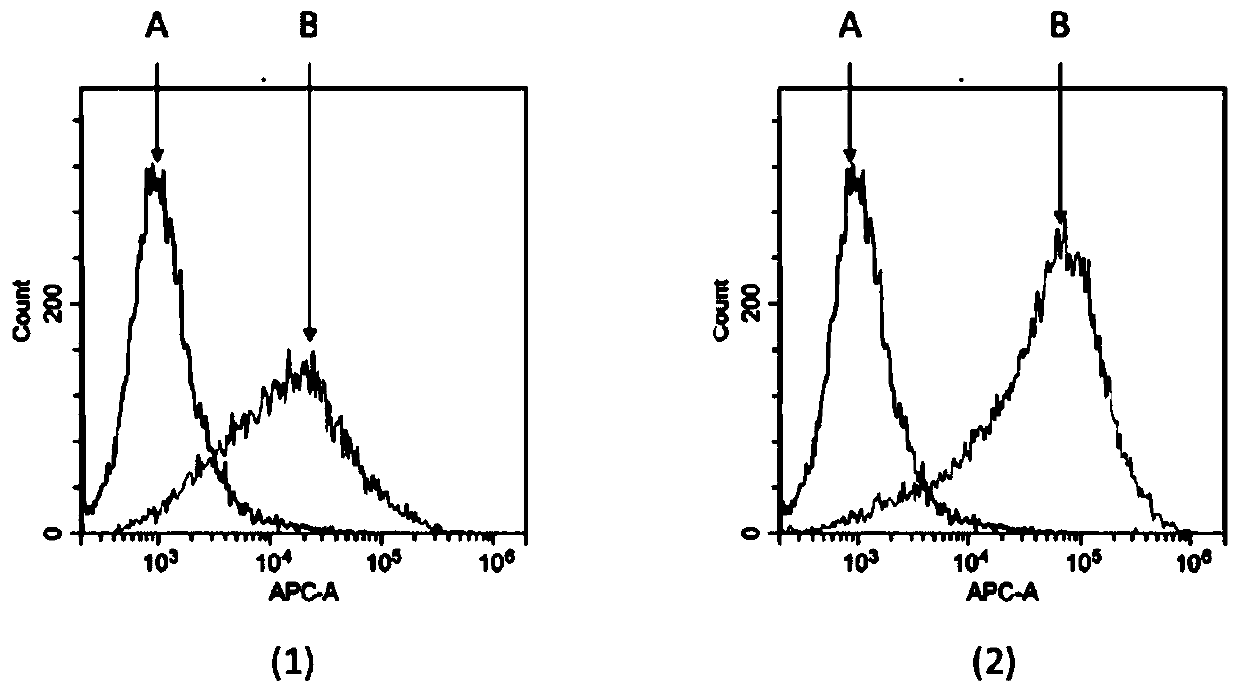
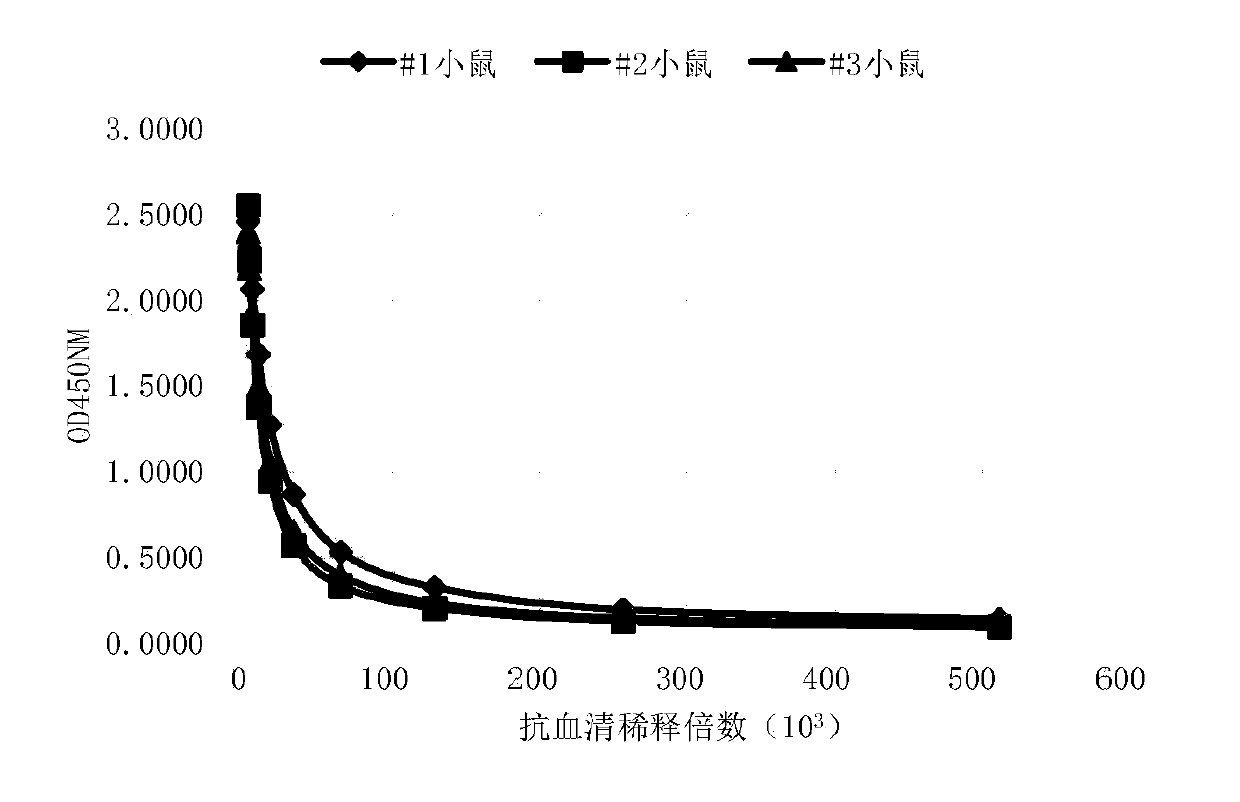
[0057] Example 2: Animal immunity and antiserum ELISA titer and flow cytometry assay
[0058] Three 6- to 8-week-old Balb / c mice (Beijing Speyford) were selected and housed in the same cage, numbered #1, #2, and #3 respectively. Four days before the first immunization, about 0.05ml of blood was collected from each mouse through the tail vein, placed at 4°C for half an hour, then centrifuged at 10,000rpm at 4°C for 10min, and the serum was separated as negative control serum for subsequent experiments. For the first immunization, the recombinant human CD70-mFc fusion protein immunogen (Acro Biosystems CDL-H525a) was fully mixed with an equal volume of Freund's complete adjuvant (SIGMA), and the phacoemulsification was complete, and two subcutaneous and two intraperitoneal injections were taken. The immunization dose For 50 μg of immunogen per mouse, the injection volume was 0.2 ml. The second and third immunizations were carried out at intervals of 14 days and 35 days respecti...
Embodiment 3
[0062] The preparation of embodiment 3 hybridoma monoclonal cell lines
[0063] 1. Cell Fusion
[0064] Cell fusion was performed using the polyethylene glycol method. The specific operation is as follows:
[0065] 1) One week before fusion, revive SP2 / 0-Ag14 myeloma cells (Beijing Beina). Two days before cell fusion, expand the culture of SP2 / 0-Ag14 so that it is in logarithmic growth phase on the day of fusion.
[0066] 2) Half an hour before cell fusion, pre-treat SP2 / 0-Ag14 cells, resuspend SP2 / 0-Ag14 cells and count, take 2-3×10 7 The cells were placed in a 37°C water bath for later use.
[0067] 3) Collect blood from the heart of the mouse to be fused, collect serum in the same manner as above, and store it at -20°C, which can be used as a positive control for screening after fusion. The mice were killed by neck dislocation, soaked in 75% alcohol, and transferred to the cell room. The spleen was taken for grinding, and filtered through a 70 μm sieve to make a singl...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com