Synthesis method of 2,3-dihydro-3,5-dihydroxy-6-methyl-4H-pyran-4-one
A synthetic method, the technology of dihydroxy, which is applied in the direction of organic chemistry, etc., can solve the problems of difficult preparation and purification, low yield, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
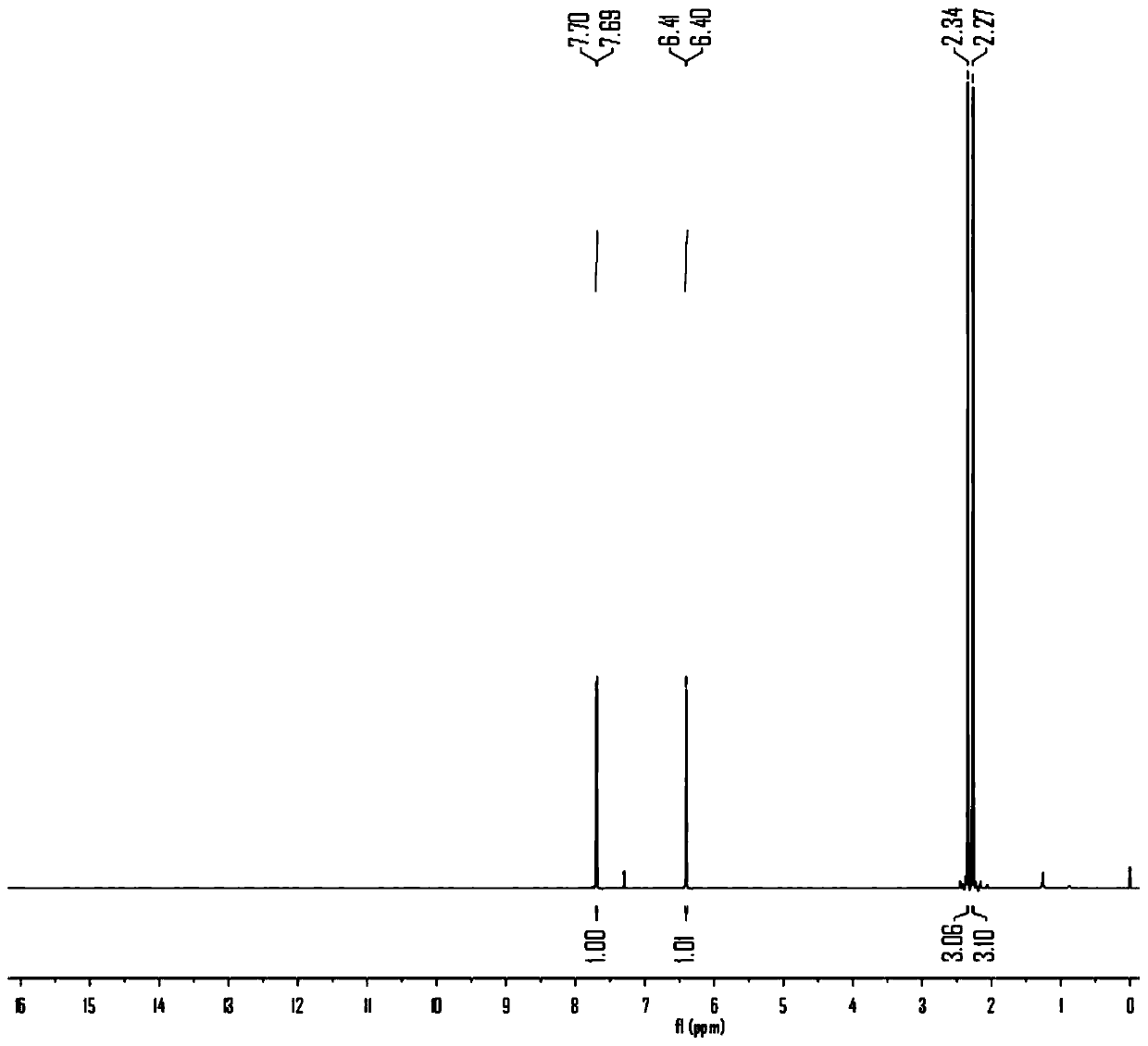
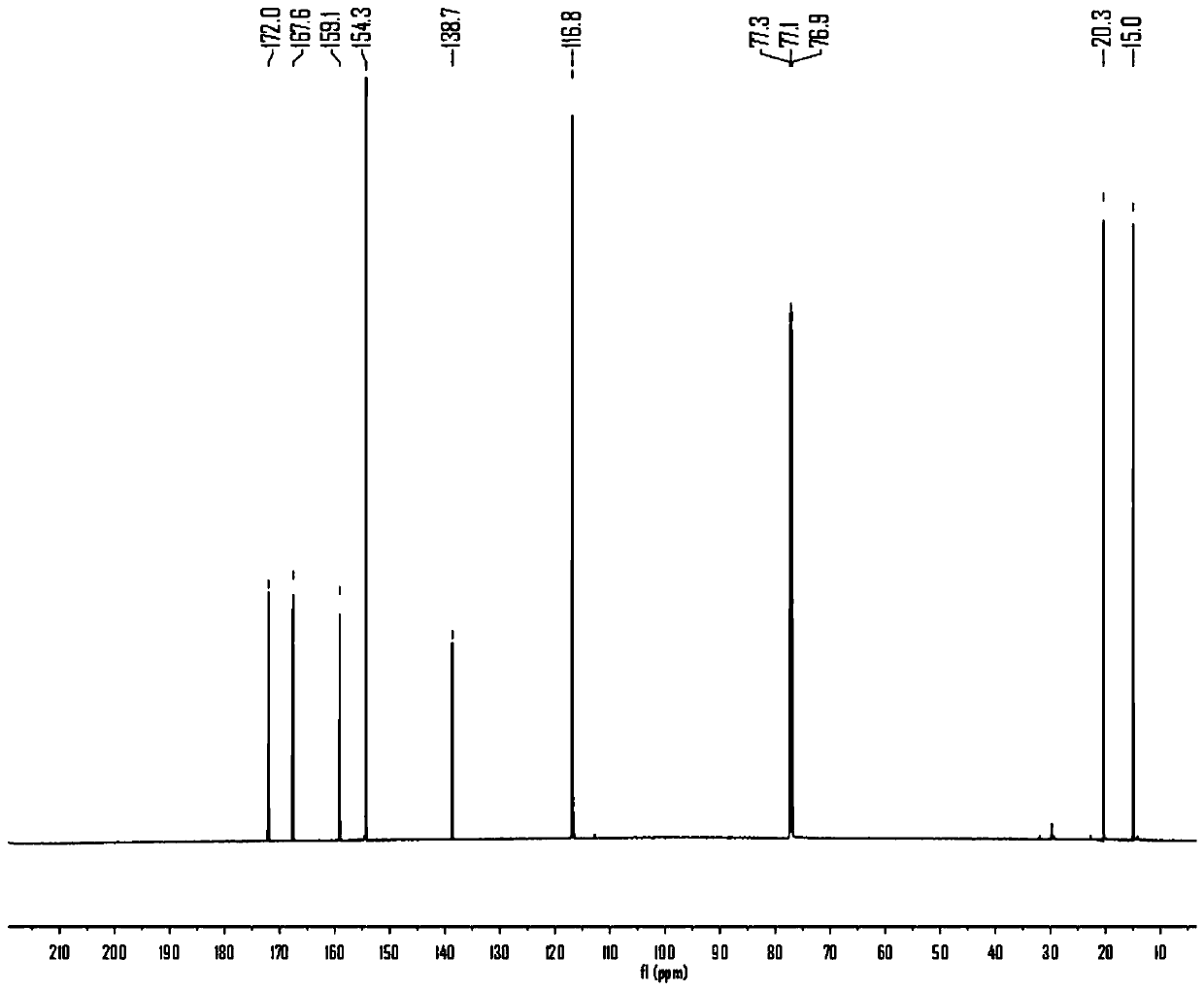
[0040] Preparation of maltol acetate: In a 100mL round bottom flask, add 5.04g maltol (40mmol), 3.93g acetyl chloride (50mmol) and 50mL absolute ethanol, react at 80°C for 4h, cool to room temperature after the reaction, Evaporate the solvent under reduced pressure at 25°C and 20 Pa, add 30 mL of distilled water, extract with dichloromethane for 3 times, combine the organic phases, and successively wash with saturated NaHCO 3 solution combined with saturated NaCl solution and washed 3 times, anhydrous NaCl 2 SO 4 After drying, the solvent was distilled off under reduced pressure to obtain 6.40 g of white solid, maltol acetate, with a yield of 95.24%.
[0041] Preparation of dihydromaltol acetate: In a 100 mL round bottom flask, add 3.36 g of maltol acetate (20 mmol), 50 mL of ethyl acetate and 0.5 g of Pd / C, replace the round bottom flask with nitrogen 3 times , replaced with hydrogen 3 times, and reacted for 6 hours at 2 times atmospheric pressure. During the period, the pr...
Embodiment 2
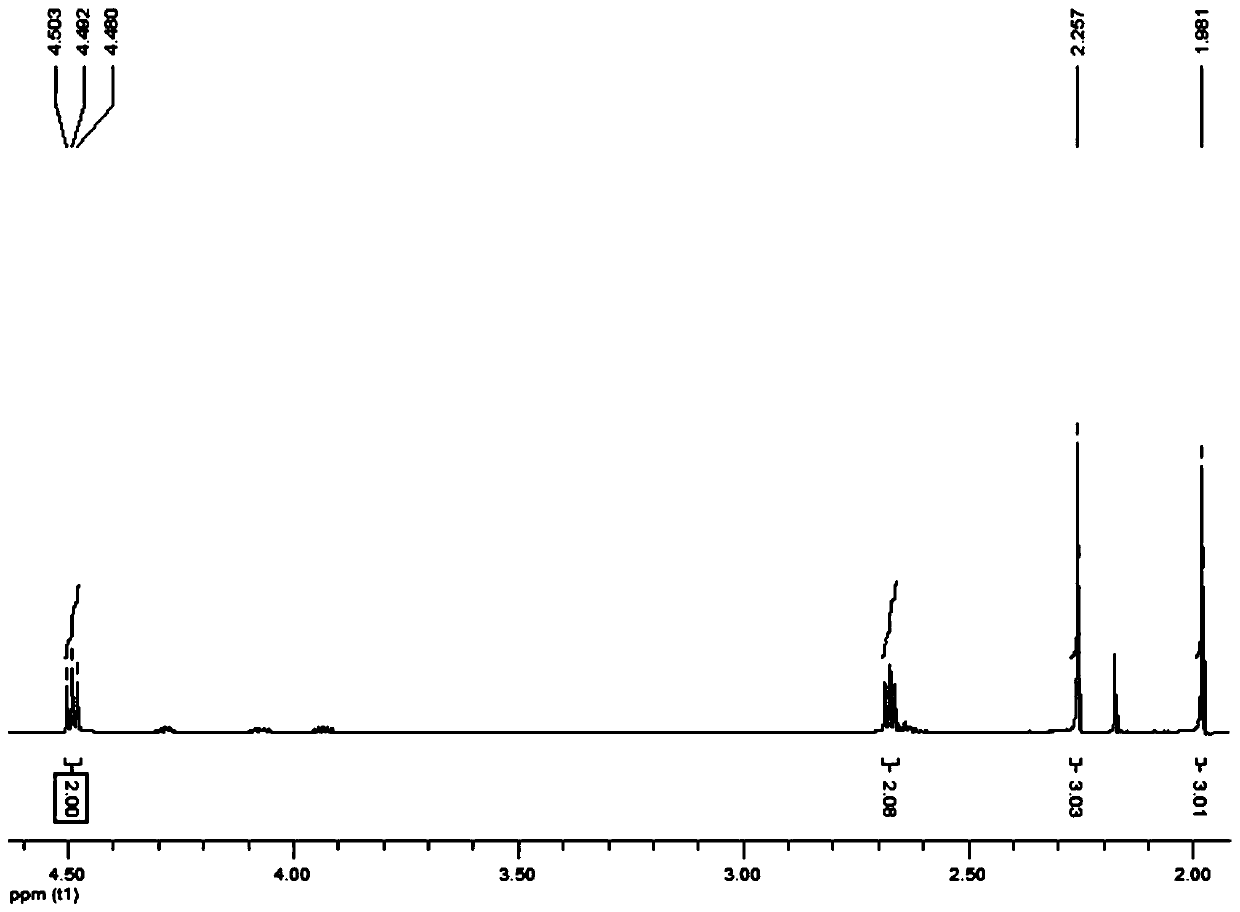
[0046] Preparation of maltol acetate: In a 100mL round bottom flask, add 5.04g maltol (40mmol), 4.71g acetyl chloride (60mmol) and 50mL absolute ethanol, react at 100°C for 6h, cool to room temperature after the reaction, Evaporate the solvent under reduced pressure at 50°C and 75Pa, add 40 mL of distilled water, extract 3 times with dichloromethane, combine the organic phases, and successively wash with saturated NaHCO 3 solution combined with saturated NaCl solution and washed 3 times, anhydrous NaCl 2 SO 4 After drying, the solvent was distilled off under reduced pressure at 50° C. and 75 Pa to obtain 6.52 g of white solid, maltol acetate, with a yield of 97.02%.
[0047] Preparation of dihydromaltol acetate: In a 100 mL round bottom flask, add 3.36 g of maltol acetate (20 mmol), 50 mL of ethyl acetate and 0.6 g of Pd / C, replace the round bottom flask with nitrogen 3 times , replaced with hydrogen 3 times, and reacted at 2 times atmospheric pressure for 8 hours. During th...
Embodiment 3
[0052] Preparation of maltol acetate: In a 100mL round bottom flask, add 5.04g maltol (40mmol), 5.10g acetic anhydride (50mmol) and 50mL absolute ethanol, react at 85°C for 6h, cool to room temperature after the reaction, Evaporate the solvent under reduced pressure at 30°C and 50Pa, add 30mL of distilled water, extract with dichloromethane for 3 times, combine the organic phases, and successively wash with saturated NaHCO 3 solution combined with saturated NaCl solution and washed 3 times, anhydrous NaCl 2 SO 4 After drying, the solvent was distilled off under reduced pressure to obtain 6.14 g of white solid, maltol acetate, with a yield of 91.37%.
[0053] Preparation of dihydromaltol acetate: In a 100mL round bottom flask, add 3.36g maltol acetate (20mmol), 50mL ethyl acetate and 0.35g nickel, replace the round bottom flask with nitrogen 3 times, hydrogen Replaced 3 times, reacted at 2 times atmospheric pressure for 8 hours, during which the pressure of the round bottom f...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com