Ph and reducing agent-responsive carrier and preparation method based on pseudorotaxane molecular structure
A technology of molecular structure and quasi-rotaxane, applied in the field of material science, can solve the problems of high release baseline, the inability of nanocarriers to achieve multiple responses, and the long time required to achieve the effect of improving species
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
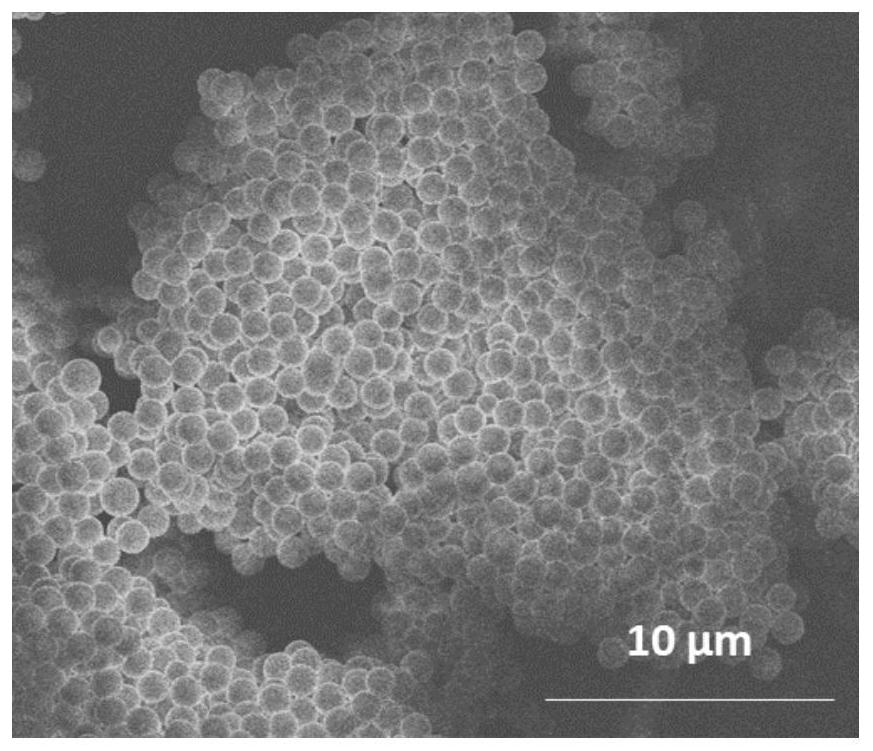
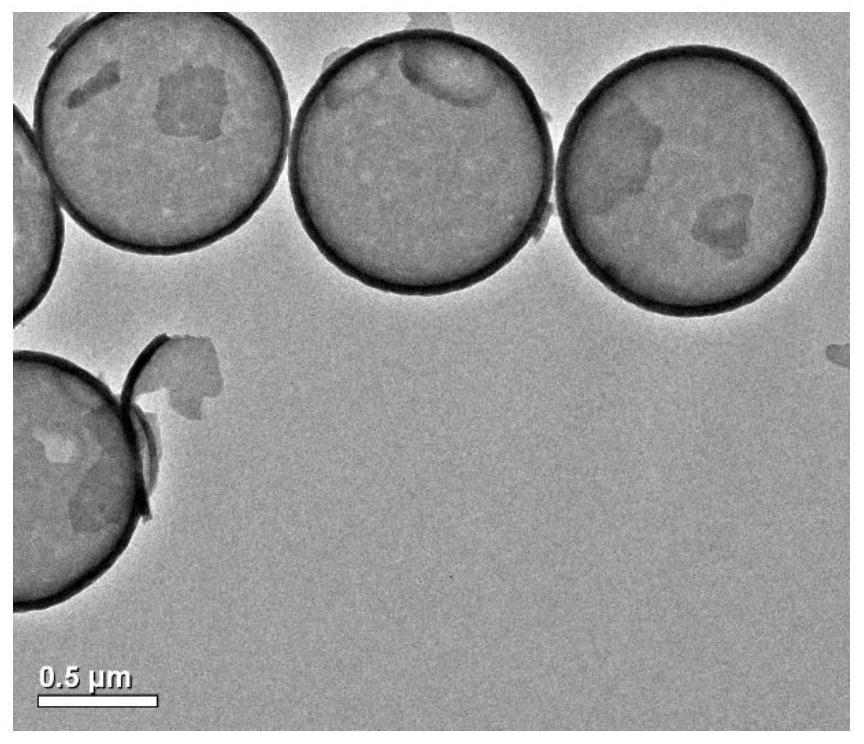
Image
Examples
Embodiment 1
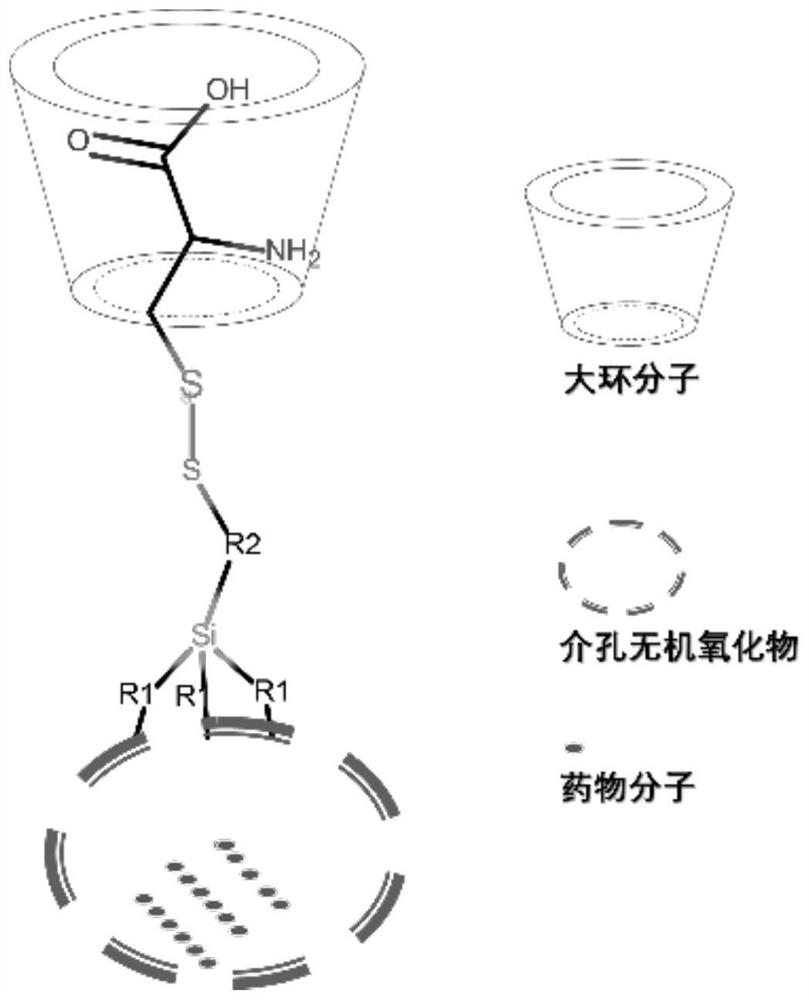
[0041] In the present invention, a multiple-response (pH, reducing agent) drug-carrying container based on the pseudorotaxane supramolecular structure comprises the following steps:
[0042] (1) Disperse 200mg of hollow silicon oxide material in xylene and heat to 100°C, then add 72.72mg of coupling agent KH590 (mercaptopropyltrimethoxysilane), and carry out surface modification reaction for 12h;
[0043] (2) 150 mg of the product obtained in step (1) was dispersed in 15% methanol aqueous solution with pH = 4.5, 75 mg of 2,2-dithiobipyridine was added, and disulfide bond exchange reaction was carried out at room temperature, and the reaction time was 4 hours;
[0044] (3) Take 100 mg of the product obtained in step (2) and disperse it in 15% methanol aqueous solution with pH = 4.5, add 100 mg of cysteine to react, and carry out disulfide bond exchange reaction at room temperature for 0.25 h;
[0045] (4) The product obtained in step (3) was dispersed in 6 g of high-concentra...
Embodiment 2
[0050] In the present invention, a multiple-response (pH, reducing agent) drug-carrying container based on the pseudorotaxane supramolecular structure comprises the following steps:
[0051] (1) Disperse 200mg of hollow zirconia material in xylene and heat to 110°C, then add 30.3mg of mercaptopropyltributoxysilane coupling agent, and carry out surface modification reaction for 16h;
[0052] (2) 150 mg of the product obtained in step (1) was dispersed in 15% methanol aqueous solution with pH = 3.5, 75 mg of 2,2-dithiobipyridine was added, and disulfide bond exchange reaction was carried out at room temperature, and the reaction time was 2 hours;
[0053] (3) Take 100 mg of the product obtained in step (2) and disperse it in 15% methanol aqueous solution with pH = 3.5, add 100 mg of cysteine to react, and carry out disulfide bond exchange reaction at room temperature for 30 min;
[0054] (4) The product obtained in step (3) was dispersed in 6 g of high-concentration rhodamine ...
Embodiment 3
[0058] In the present invention, a multiple-response (pH, reducing agent) drug-carrying container based on the pseudorotaxane supramolecular structure comprises the following steps:
[0059] (1) Disperse 200mg of hollow silica material in toluene and heat to 80°C, then add 150mg of mercaptopropyldimethoxysilane, and carry out surface modification reaction for 24 hours;
[0060] (2) 150 mg of the product obtained in step (1) was dispersed in 15% methanol aqueous solution with pH=5.5, 75 mg of 2,2-dithiobipyridine was added, and disulfide bond exchange reaction was carried out at room temperature, and the reaction time was 6 hours;
[0061] (3) Take 100 mg of the product obtained in step (2) and disperse it in 15% methanol aqueous solution with pH = 5.5, add 100 mg of cysteine to react, and carry out disulfide bond exchange reaction at room temperature for 2 h;
[0062] (4) The product obtained in step (3) is dispersed in 6 g of high-concentration rhodamine drug aqueous soluti...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com