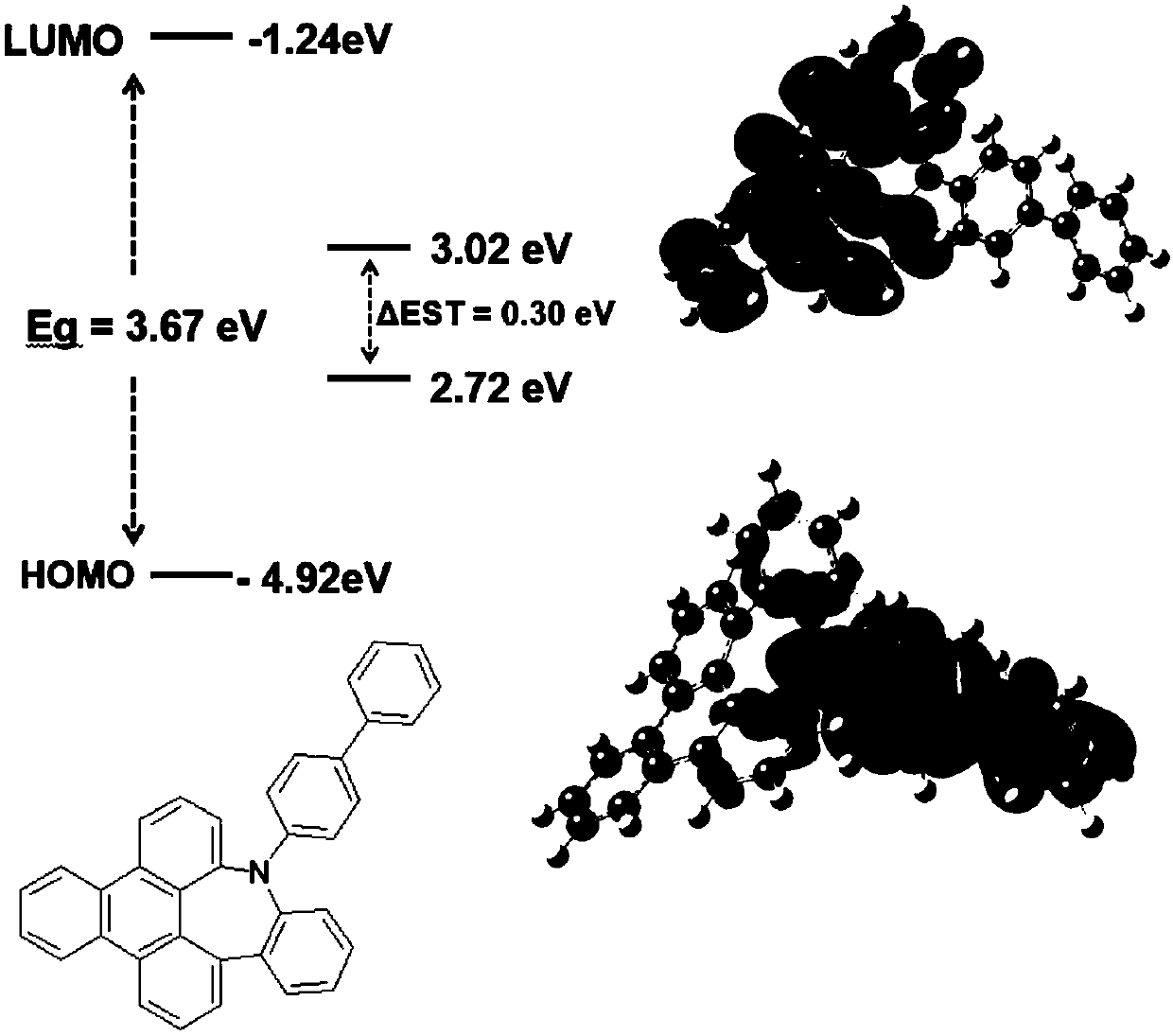
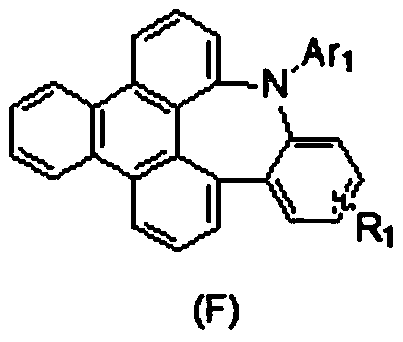
Tri-hypo-phenanthrene nitrogen-containing seven-membered ring compound, preparation method therefor and application of tri-hypo-phenanthrene nitrogen-containing seven-membered ring compound
A compound and seven-membered ring technology, which is applied in the field of Sanya phenanthrene nitrogen-containing seven-membered ring compound and its preparation, can solve problems affecting performance and difficulty in forming a uniform film, and achieve high glass transition temperature, easy access, and reaction The effect of regulation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0044] This embodiment provides a nitrogen-containing seven-membered ring compound of Sanyaphenanthrene, which has the structure shown in the following formula F-1:
[0045]
[0046] The synthetic route of compound shown in formula F-1 is as follows:
[0047]
[0048] The preparation method of the compound shown in formula F-1 specifically comprises the following steps:
[0049] (1) Preparation of intermediate (C-1)
[0050] Under nitrogen protection, in a 500ml four-necked flask, compound (A) 22.9g (84mmol), compound (B-1) 14.1g (70mmol), Pd (PPh 3 ) 4 4g, (34.6mmol), Na 2 CO 3 22.3g (210mmol) was dissolved in a mixture of 200mL of toluene, 50mL of EtOH, and 50mL of distilled water, refluxed and stirred at 120°C for 6 hours, then quenched the reaction with water, separated the water phase, removed most of the solvent by rotary evaporation, precipitated solid and dried to obtain the intermediate Compound (C-1) 24.1g (98% yield);
[0051] (2) Preparation of Intermed...
Embodiment 2
[0057] This embodiment provides a nitrogen-containing seven-membered ring compound of Sanyaphenanthrene, which has the structure shown in the following formula F-2:
[0058]
[0059] The synthetic route of compound shown in formula F-2 is as follows:
[0060]
[0061] The preparation method of the compound shown in formula F-2 specifically comprises the following steps:
[0062] (1) Preparation of intermediate (C-1)
[0063] The preparation of intermediate (C-1) is the same as in Example 1;
[0064] (2) Preparation of Intermediate (D-1)
[0065] The preparation of intermediate (D-1) is the same as in Example 1;
[0066] (3) Preparation of compound (F-2)
[0067] Under nitrogen protection, in a 500ml four-necked flask, add 19g (60mmol) compound (D-1) at room temperature, 17.7g (65mmol) compound (E-2), 200ml of toluene, 11.5g (120mmol) sodium tert-butoxide , 182 mg (0.2 mmol) Pd 2 (dba) 3 , 1.2ml (0.6mmol) 10% tri-tert-butylphosphine toluene solution, after reflux r...
Embodiment 3
[0070] This embodiment provides a nitrogen-containing seven-membered ring compound of Sanyaphenanthrene, which has the structure shown in the following formula F-3:
[0071]
[0072] The synthetic route of compound shown in formula F-3 is as follows:
[0073]
[0074] The preparation method of the compound shown in formula F-3 specifically comprises the following steps:
[0075] (1) Preparation of intermediate (C-1)
[0076] The preparation of intermediate (C-1) is the same as in Example 1;
[0077] (2) Preparation of Intermediate (D-1)
[0078] The preparation of intermediate (D-1) is the same as in Example 1;
[0079] (3) Preparation of Compound (F-3)
[0080] Under nitrogen protection, in a 500ml four-necked flask, add 19g (60mmol) compound (D-1) at room temperature, 21g (65mmol) compound (E-3), 200ml of toluene, 11.5g (120mmol) sodium tert-butoxide, 182mg (0.2mmol) Pd 2 (dba) 3 , 1.2ml (0.6mmol) 10% tri-tert-butylphosphine toluene solution, after reflux reacti...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com