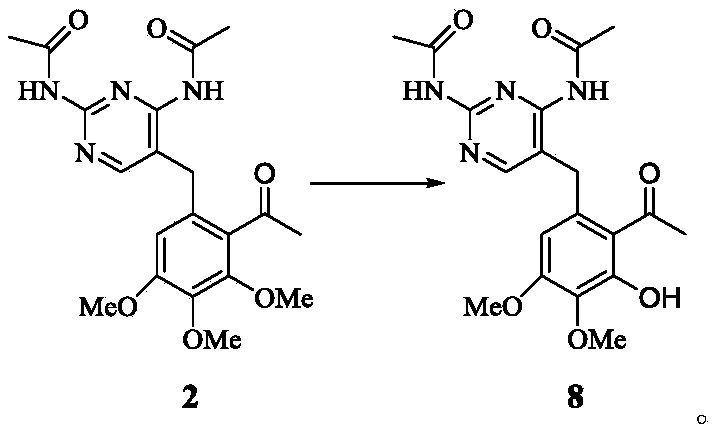
Iclaprim intermediate, and preparation method for iclaprim
A scheme and compound technology, applied in the field of alaprime intermediates and their preparation, can solve the problems of low yield, long route, expensive reagents, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
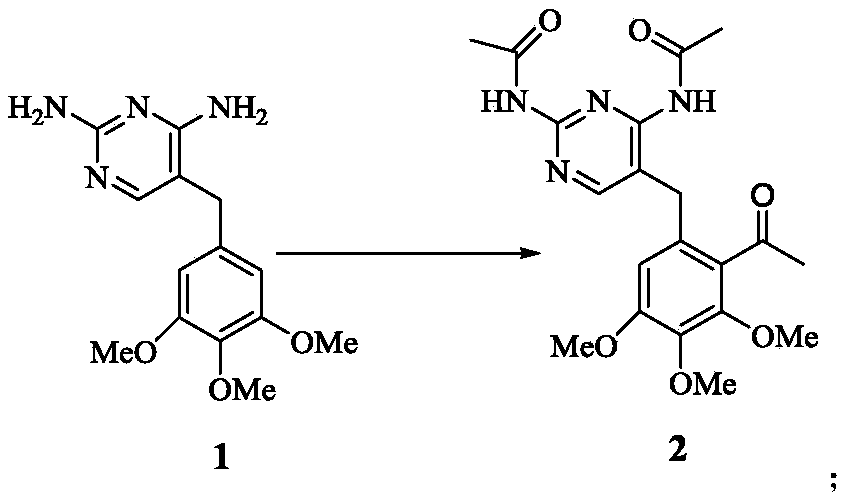
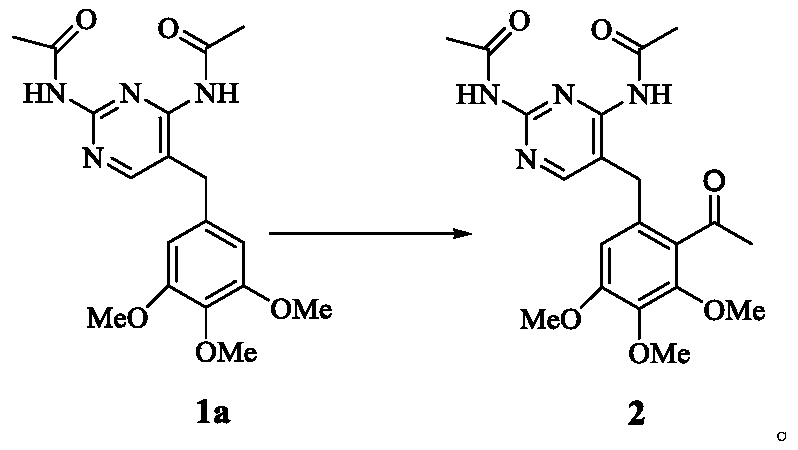
[0115] Preparation of Compound 1a (N,N'-(5-(3,4,5-trimethoxybenzyl)pyrimidine-2,4-diyl)diacetamide)
[0116]
[0117] Add compound 1 (100.0g, 344.5mmol), acetic anhydride (176g, 1710.4mmol) and 900ml toluene to the reaction flask, heat to reflux for 1.5h, let it stand for crystallization after cooling down to room temperature, suction filter, and dry to obtain 109.0g White solid 1a, the yield is 84.5%, mp.201-203°C, the purity is 98.72% by HPLC, 1 H-NMR (400MHz, CDCl 3 ).δ(ppm):10.41(s,1H),10.09(s,1H),8.36(s,1H),6.48(s,1H),6.34(s,2H),3.84(s,2H),3.71 (s,6H), 3.61(s,3H), 2.16(s,6H).
Embodiment 2
[0119] Preparation of Compound 1a (N, N'-(5-(3,4,5-trimethoxybenzyl)pyrimidine-2,4-diyl)diacetamide)
[0120] Add compound 1 (100.1g, 344.8mmol), acetyl chloride (98.1ml, 1379.6mmol) and 900ml toluene to the reaction flask, heat and reflux and stir for 1.5h, after cooling down to room temperature, stand for crystallization, filter with suction, and dry to obtain 104.0 g white solid 1a, yield 80.6%, mp.201-203 ° C, HPLC detection of its purity is 98.70%, 1 H-NMR (400MHz, CDCl 3 ).δ(ppm):10.41(s,1H),10.09(s,1H),8.36(s,1H),6.48(s,1H),6.34(s,2H),3.84(s,2H),3.71 (s,6H), 3.61(s,3H), 2.16(s,6H).
Embodiment 3
[0122] Preparation of Compound 1a (N,N'-(5-(3,4,5-trimethoxybenzyl)pyrimidine-2,4-diyl)diacetamide)
[0123] Add compound 1 (100.0g, 344.5mmol), acetic anhydride (70.3g, 688.5mmol) and 900ml toluene to the reaction flask, heat to reflux for 1.5h, let it stand for crystallization after cooling down to room temperature, suction filter, and dry to obtain 102.8 g white solid 1a, yield 71.7%, mp.201-203°C, HPLC detected its purity as 98.62%, 1 H-NMR (400MHz, CDCl 3 ).δ(ppm):10.41(s,1H),10.09(s,1H),8.36(s,1H),6.48(s,1H),6.34(s,2H),3.84(s,2H),3.71 (s,6H), 3.61(s,3H), 2.16(s,6H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com