Preparation method of N-benzyl-3-oxopiperidine-4-carboxylic acid ethyl ester hydrochloride
A technology of ethyl carboxylate hydrochloride and oxopiperidine, applied in the field of preparation of ethyl N-benzyl-3-oxopiperidine-4-carboxylate hydrochloride, can solve the problem of low product purity problem, to achieve the effect of high product yield, high purity and low cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0018] Embodiment 1: Preparation of ethyl N-benzyl-3-oxopiperidine-4-carboxylate hydrochloride
[0019] Follow the steps below:
[0020] (1) Preparation of intermediate 2
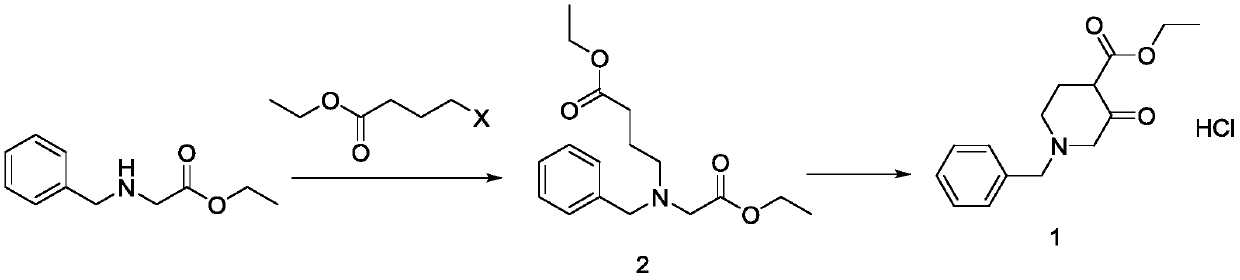
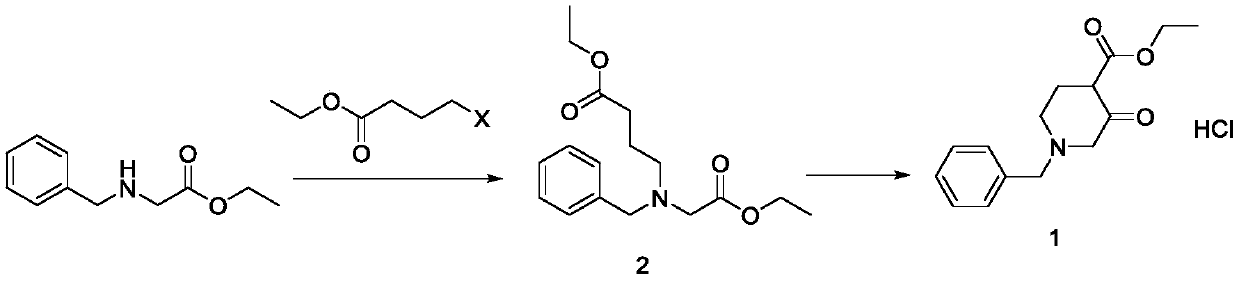
[0021] In a 20L four-necked reaction flask, add 6kg (31mol) of ethyl N-benzylglycine, add 6.65kg (34.1mol) of ethyl 4-bromobutyrate, add 6L of methanol, 3.6kg (34.1mol) of sodium carbonate, and heat to Reflux, heat preservation and reflux reaction for 10 hrs, after the basic reaction was detected by TLC, the reaction was stopped, the reaction liquid was cooled to room temperature, filtered, and the filtrate was concentrated under reduced pressure to obtain 29.41 kg of an intermediate with a yield of 98.6%.
[0022] (2) Preparation of crude product
[0023] Add the intermediate 2 prepared in the previous step into a 20L four-necked reaction flask, add 10L of ethyl acetate, add 1.84kg (34.1mol) of sodium methoxide, heat to reflux, keep warm and reflux for 4hrs, and stop the reaction after the basic reaction...
Embodiment 2
[0026] Embodiment 2: Preparation of ethyl N-benzyl-3-oxopiperidine-4-carboxylate hydrochloride
[0027] Follow the steps below:
[0028] (1) Preparation of intermediate 2
[0029] Add 6kg (31mol) of N-benzylglycine ethyl ester into a 20L four-necked reaction flask, add 7kg (46.5mol) of ethyl 4-chlorobutyrate, add 6L of toluene, 6.43kg (46.5mol) of potassium carbonate, and heat to reflux , heat-preserved and refluxed for 8 hours, after the basic reaction was detected by TLC, the reaction was stopped, the reaction liquid was cooled to room temperature, filtered, and the filtrate was concentrated under reduced pressure to obtain 29.46 kg of intermediate, with a yield of 99.1%.
[0030] (2) Preparation of crude product
[0031] Add the intermediate 2 prepared in the previous step into a 20L four-necked reaction flask, add 10L of toluene, add 5.22kg (46.5mol) of potassium tert-butoxide, heat to reflux, keep warm and reflux for 3hrs, and stop the reaction after the basic reaction ...
Embodiment 3
[0034] Embodiment 3: Preparation of ethyl N-benzyl-3-oxopiperidine-4-carboxylate hydrochloride
[0035] Follow the steps below:
[0036] (1) Preparation of Intermediate 2
[0037] Add 6kg (31mol) of N-benzylglycine ethyl ester into a 20L four-necked reaction flask, add 7.86kg (40.3mol) of ethyl 4-bromobutyrate, add 6L of ethanol, 1.61kg (40.3mol) of sodium hydroxide, and heat To reflux, keep warm and reflux for 12 hrs, after TLC detects that the basic reaction is complete, stop the reaction, cool the reaction solution to room temperature, filter, and concentrate the filtrate under reduced pressure to obtain 9.4kg of intermediate 2 with a yield of 98.5%
[0038] (2) Preparation of crude product
[0039] Add the intermediate 2 prepared in the previous step into a 20L four-necked reaction flask, add 10L of tetrahydrofuran, add 2.74kg (40.3mol) of sodium ethoxide, heat to reflux, keep warm and reflux for 4hrs, and stop the reaction after TLC detects that the basic reaction is co...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com