Hydrazide-containing quinazolinone derivative, and preparation method and application thereof
A technology of quinazolinone and derivatives, applied in the field of pesticides, can solve problems such as environmental pollution, single plant pathogenic fungus resistance, non-target biological toxicity, etc., and achieve the effect of obvious application value
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
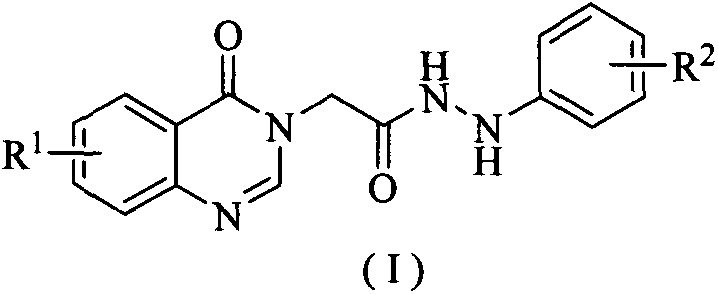
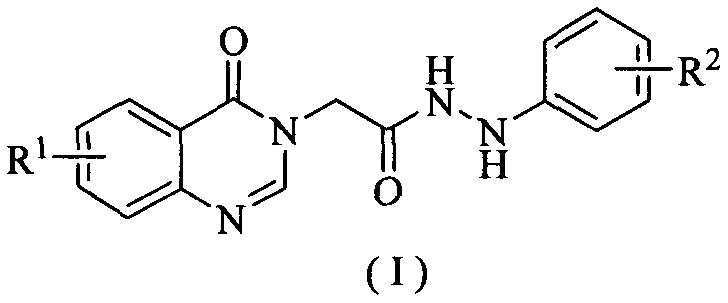
[0039] Example 1: Synthesis of 2-(4-oxoquinazolin-3(4H)-yl)-N'-(4-fluorophenyl)acethydrazine (I1)
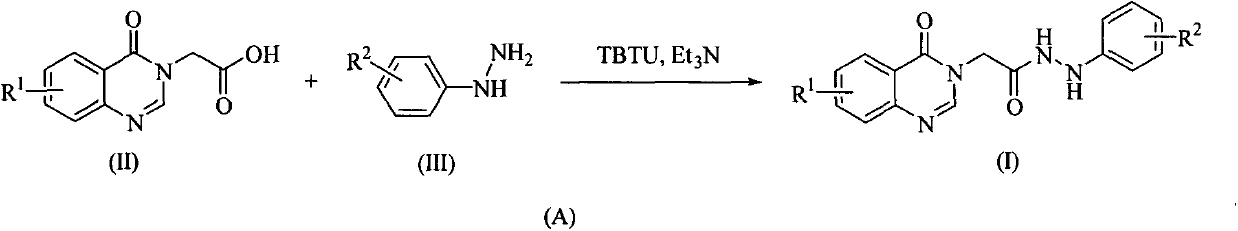
[0040] Combine 2-(4-oxoquinazoline-3(4H)-yl)acetic acid (II-1) (2.50mmol), 4-fluorophenylhydrazine (III-1) (2.50mmol), triethylamine (7.50mmol) mmol), O-benzotriazole-N,N,N',N'-tetramethyluronium tetrafluoroborate (TBTU) (2.50mmol) and acetonitrile (30mL) were added to a 50mL three-necked flask, After stirring for 4 hours at room temperature, the reaction was stopped. The reaction solution was filtered, the filter cake was washed with ethanol and dichloromethane successively, and dried to obtain the compound 2-(4-oxoquinazolin-3(4H)-yl)-N'-(4-fluorophenyl ) Acetylhydrazine (I1).
[0041]
[0042] Compounds I2-I21 were synthesized sequentially according to the method in Example 1. The structures of the synthesized hydrazide-containing quinazolinone derivatives (I1-I21) all adopted infrared spectroscopy (IR), nuclear magnetic resonance spectroscopy (NMR) and high Resolved mass spec...
Embodiment 2
[0065] Example 2: The bactericidal activity of a hydrazide-containing quinazolinone derivative I1-I21 of formula (I) of the present invention
[0066] The mycelial growth rate method was used to determine the inhibitory activity of a quinazolinone derivative I1-I21 containing hydrazide structure against Rhizoctonia solani and Fusarium graminearum. The specific operation Proceed as follows:
[0067] 1. Weigh a certain amount of target compound and dissolve it in a certain volume of dimethyl sulfoxide to obtain a certain mass concentration of mother liquor;
[0068] 2. Measure 100μL of mother liquor and add it to 45mL of autoclaved potato agar medium, shake evenly;
[0069] 3. Pour the above-mentioned medium evenly into 3 petri dishes with a diameter of 9 cm, and after it has solidified, transfer the fungus cake with a diameter of 5 mm to the center of the petri dish;
[0070] 4. After the above-mentioned petri dish is cultured at 25±1°C until the colony is about 7.0 to 7.5 cm directly...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com