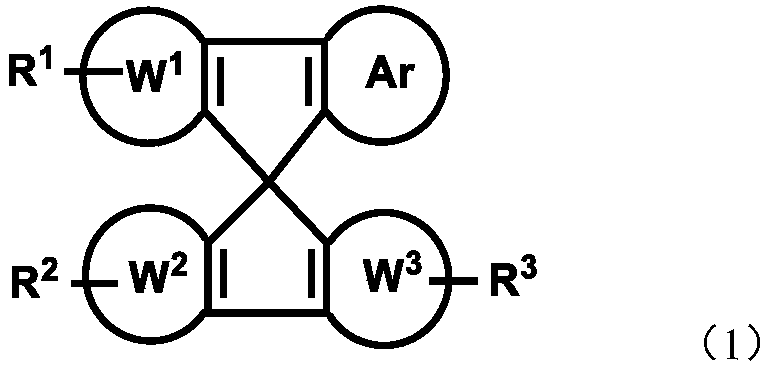
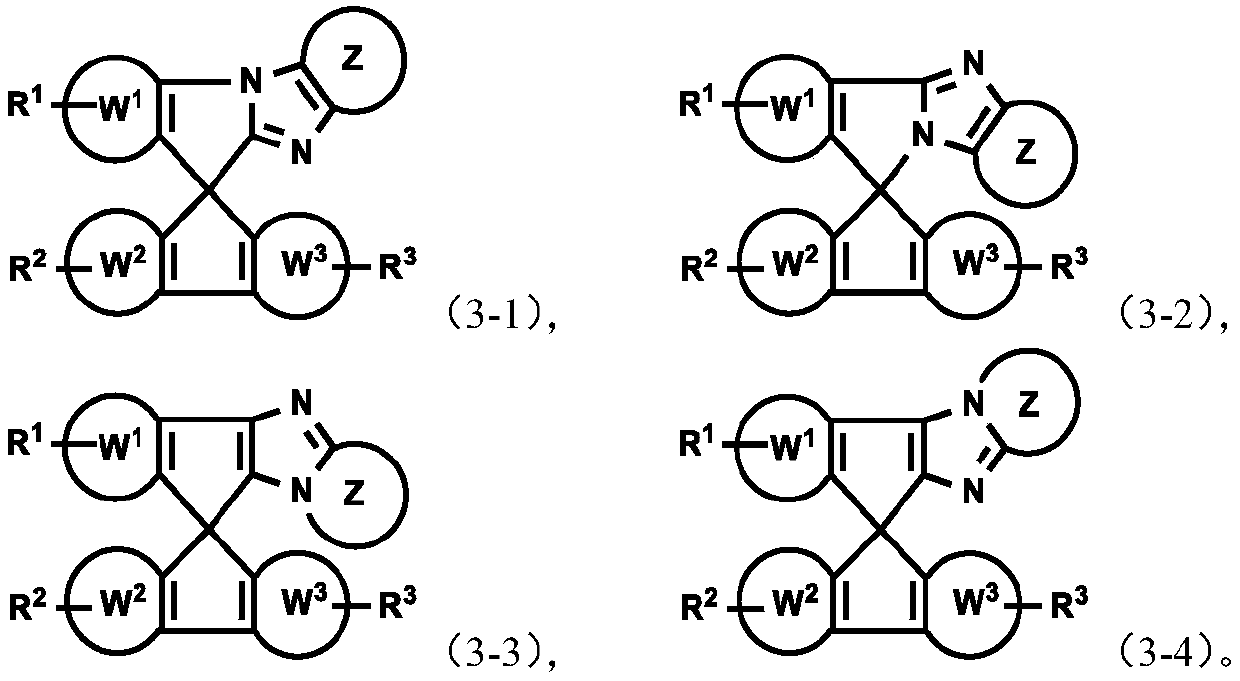
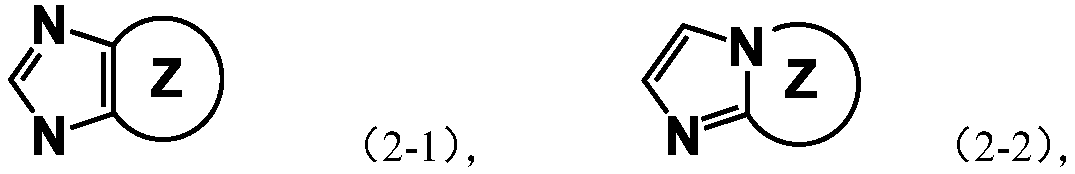Imidazole spiro-containing compound and application thereof
An imidazole spiro and compound-containing technology is applied in the fields of mixtures, compositions and organic electronic devices, compounds containing imidazole spiro rings, and polymers, and can solve the problems of low performance of organic electronic components and devices, and achieve good transmission capacity. , the effect of optimizing device performance, improving efficiency and life
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0174] Example 1: Synthesis of compound (M1):
[0175]
[0176] synthetic route:
[0177]
[0178] 1) Synthesis of Intermediate M1-3: Under nitrogen atmosphere, (33.6g, 100mmol) of compound M1-1, (24.4g, 200mmol) of M1-2, (6.9g, 6mmol) tetrakis(triphenylphosphonium) ) Palladium, (6.5g, 20mmol) tetrabutylammonium bromide, (8g, 200mmol) sodium hydroxide, (40 mL) water and (250mL) toluene were added to a 500mL three-neck flask, heated at 80°C and stirred for 12 hours, After finishing the reaction, the reaction solution was rotated to evaporate most of the solvent, dissolved in dichloromethane, washed with water 3 times, and the organic solution was collected and mixed with silica gel for purification. The yield was 80%.
[0179] 2) Synthesis of intermediate M1-6: under a nitrogen atmosphere, combine (12.4g, 100mmol) of compound M1-4, (17.3g, 100mmol) of compound M1-5, (27.6g, 200mmol) of potassium carbonate and 300mL Dimethylformamide was added to a 500mL three-necked flask, heated to...
Embodiment 2
[0182] Example 2: Synthesis of Compound (M2):
[0183]
[0184] synthetic route:
[0185]
[0186] 1) Synthesis of intermediate M2-2: According to the synthesis method of compound M1-6, compound M2-1 was substituted for compound M1-4, and the yield was 85%.
[0187] 2) Synthesis of intermediate M2-4: According to the synthesis method of compound M1-7, compound M2-2 and compound M2-3 are substituted for compounds M1-6 and M1-3.
[0188] 3) Synthesis of intermediate M2-5: According to the synthesis method of compound M1, compound M2-4 is substituted for compound M1-7, and the yield of the two steps is 70%.
[0189] 4) Synthesis of Intermediate M2-6: Under nitrogen atmosphere, (21.7g, 50mmol) of compound M2-5, (12.7g, 50mmol) pinacol diborate, (9.8g, 100mmol) potassium acetate, ( 2.2g, 3mmol)Pd(ppf)Cl 2 Add 150mL of 1,4-dioxane as a solvent to a 250mL three-necked flask, heat at 110°C to react for 12 hours, after the reaction is complete, the reaction solution is cooled to room temperatur...
Embodiment 3
[0191] Example 3: Synthesis of Compound (M3):
[0192]
[0193] synthetic route:
[0194]
[0195] 1) Synthesis of intermediate M3-2: According to the synthesis method of compound M1-6, compound M3-1 was substituted for compound M1-4, and the yield was 80%.
[0196] 2) Synthesis of intermediate M3-3: According to the synthesis method of compound M1-7, compound M3-2 and compound M1-1 are substituted for compound M1-6 and M1-3.
[0197] 3) Synthesis of intermediate M3-4: According to the synthesis method of compound M1, compound M3-3 is substituted for compound M1-7, and the yield of the two steps is 65%.
[0198] 4) Synthesis of compound M3: According to the synthesis method of compound M1-3, compounds M3-4 and M3-5 were substituted for compounds M1-1 and M1-2, and the yield was 75%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| glass transition temperature | aaaaa | aaaaa |
| glass transition temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com