9S-acyloxy cinchonine derivatives, preparation method and application thereof, and plant-derived insecticide
A technology of acyloxy cinchonines and derivatives, which is applied in the field of botanical insecticides, can solve problems such as unreported research, and achieve the effects of simple operation, mild reaction conditions, and good insecticidal effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 14
[0049] This embodiment is the preparation method of compound 3a in embodiment 1, specifically comprising the following steps:
[0050] (1) Weigh 0.5mmol of cinchonine (1), 0.6mmol of glacial acetic acid (2a), 0.6mmol of DCC and 0.1mmol of DMAP in a 50mL flask and mix well, then add 10mL of calcium hydride dried Dichloromethane, react at room temperature after mixing evenly, adopt TLC tracking detection in the reaction process, the reaction ends after the raw material reaction is complete;
[0051](2) Then remove urea by filtration to obtain the filtrate; the filtrate is diluted with 40 mL of dichloromethane, and then the diluted solution is sequentially washed with 0.1mol / L hydrochloric acid (25 mL), saturated sodium bicarbonate solution (25 mL) and saturated Brine (25mL) was washed successively, and then dried with anhydrous sodium sulfate; the dried dilution was distilled off under reduced pressure to remove the solvent to obtain a solid, and then the solid was separated by ...
Embodiment 15
[0057] This example is the preparation method of compound 3b, specifically refer to the preparation process of Example 14, the only difference is: replace glacial acetic acid (2a) with n-hexanoic acid (2b), the reaction involved is:
[0058]
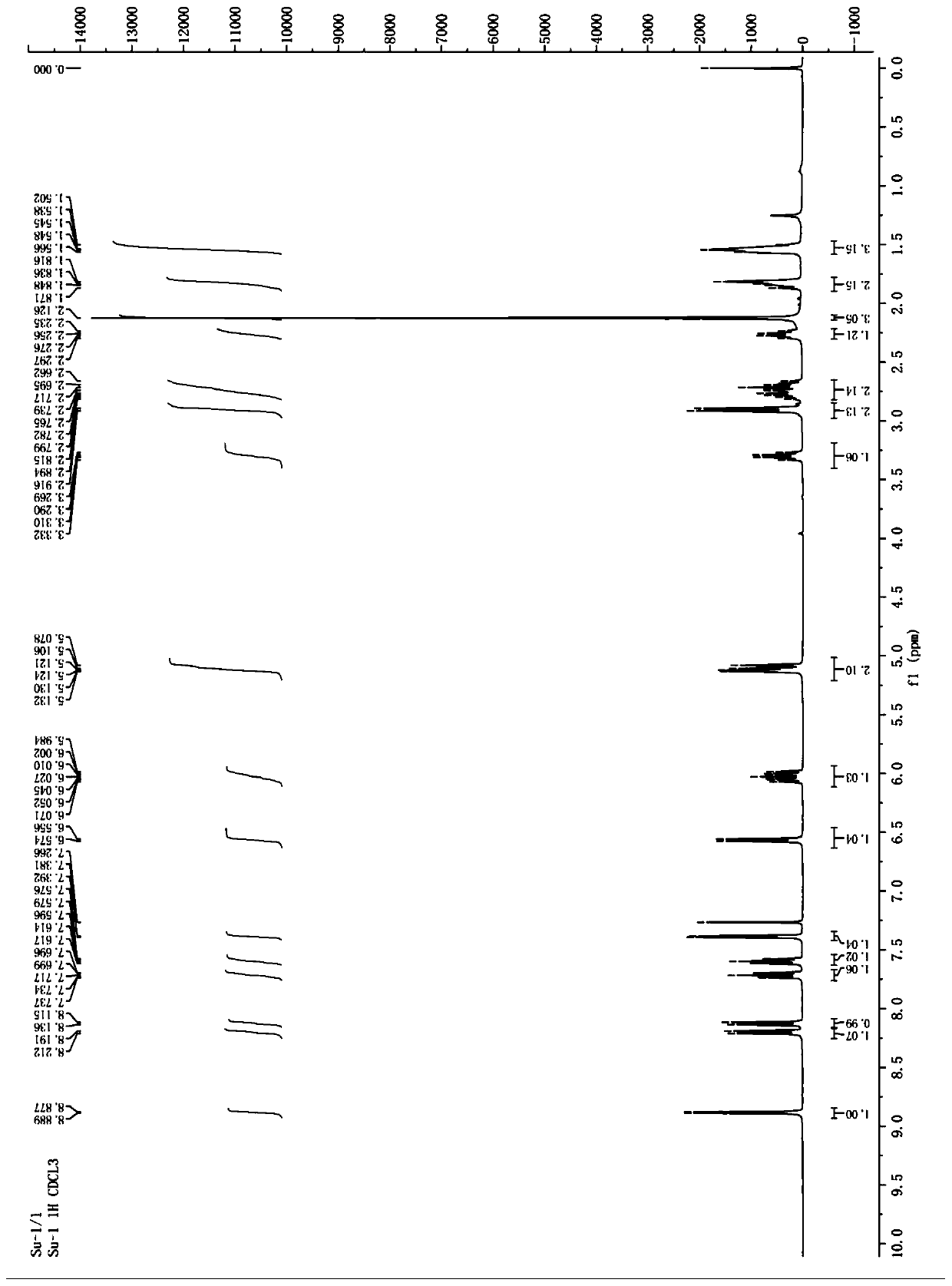
[0059] The yield of compound 3b prepared by the preparation method of this example was 39%. Carry out 400MHz proton nuclear magnetic resonance spectrum test to the obtained compound 3b, the solvent that adopts during the test is CDCl 3 , TMS is the internal standard, and the test results are as follows figure 2 Shown, the specific chemical shift δ of each peak is: 8.88 (d, J=4.4Hz, 1H), 8.22 (dd, J=8.8Hz, 1.2Hz, 1H), 8.13 (dd, J=8.4Hz, 1.2Hz , 1H), 7.69-7.73(m, 1H), 7.57-7.61(m, 1H), 7.38(d, J=4.4Hz, 1H), 6.56(d, J=7.2Hz, 1H), 5.98-6.07( m, 1H), 5.07-5.13(m, 2H), 3.33(q, J=8.4Hz, 1H), 2.85-2.95(m, 2H), 2.65-2.80(m, 2H), 2.32-2.41(m, 2H), 2.22-2.29(m, 1H), 1.80-1.85(m, 2H), 1.51-1.58(m, 3H), 1.19-1.29(m, 4H), 0.89-0.98(m, 2H), 0.86...
Embodiment 16
[0062] This example is the preparation method of compound 3c, specifically refer to the preparation process of Example 14, the only difference is: replace glacial acetic acid (2a) with benzoic acid (2c), the reaction involved is:
[0063]
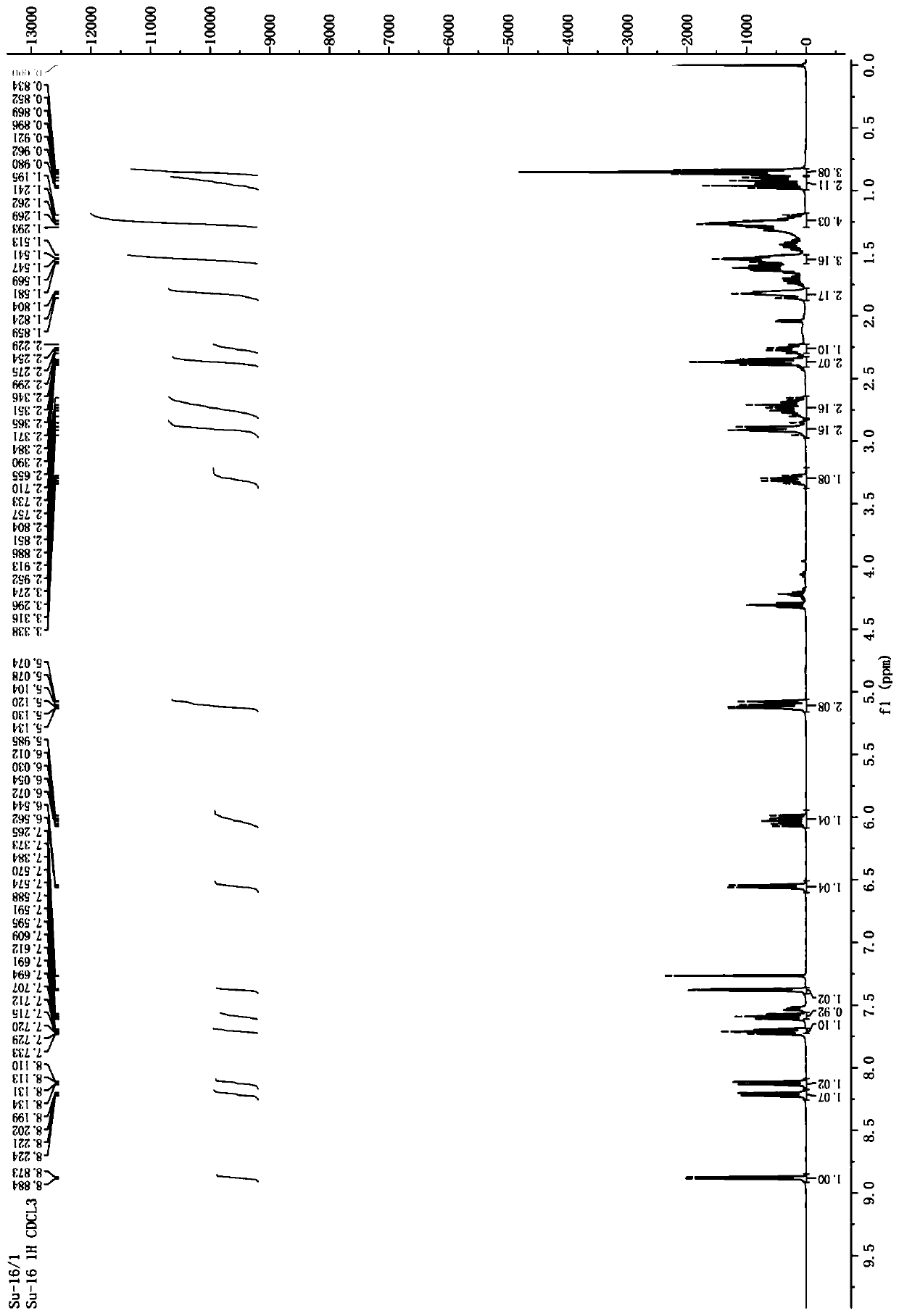
[0064] The yield of compound 3c prepared by the preparation method of this example was 54%. Carry out 400MHz proton nuclear magnetic resonance spectrum test to the obtained compound 3c, the solvent that adopts during the test is CDCl 3 , TMS is the internal standard, and the test results are as follows image 3 Shown, the specific chemical shift δ of each peak is: 8.87 (d, J=4.4Hz, 1H), 8.32 (dd, J=8.4Hz, 1.2Hz, 1H), 8.08-8.14 (m, 3H), 7.70- 7.74(m, 1H), 7.57-7.65(m, 2H), 7.44-7.48(m, 3H), 6.80(d, J=7.2Hz, 1H), 5.98-6.07(m, 1H), 5.06-5.13( m, 2H), 3.48(q, J=8.4Hz, 1H), 2.90-3.03(m, 2H), 2.68-2.85(m, 2H), 2.25-2.32(m, 1H), 1.94-2.01(m, 1H), 1.85-1.87 (m, 1H), 1.56-1.69 (m, 3H).
[0065] The high resolution chromatography (HRMS, ESI) t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com