Plasminogen measuring kit and preparation method thereof
A technology of plasmin and kits, which is applied in the direction of measuring devices, instruments, scientific instruments, etc., can solve the problems of low detection accuracy and sensitivity, and achieve the effects of convenient instrument measurement, stable environment, and increased turbidity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0049] Reagent R1 includes the following raw materials:
[0050] 100mmol / L citrate buffer;
[0051] 10mmol / L sodium azide;
[0052] PEG 6000 at 0.8 mmol / l;
[0053] 2g / L stachyose;
[0054] 1.6g / L sodium fructose diphosphate;
[0055] 0.25g / L sodium hexametaphosphate;
[0056] 50mmol / L of sodium chloride;
[0057] 5g / L bovine serum albumin BSA;
[0058] The reagent R2 comprises the following raw materials:
[0059] 100mmol / L citrate buffer;
[0060] 0.85% (by mass volume ratio) of latex particles;
[0061] 10mmol / L sodium azide;
[0062] 1.8mg / mL goat anti-human plasminogen antibody;
[0063] 5g / L bovine serum albumin BSA.
[0064]The preparation method is as follows: it includes the following steps: step S1, weighing each component of the reagent R1, stirring evenly, standing still, filtering and removing impurities; Add other components. Step S2, weighing each component of the reagent R2, stirring evenly, standing still, filtering and removing impurities; in the...
Embodiment 2
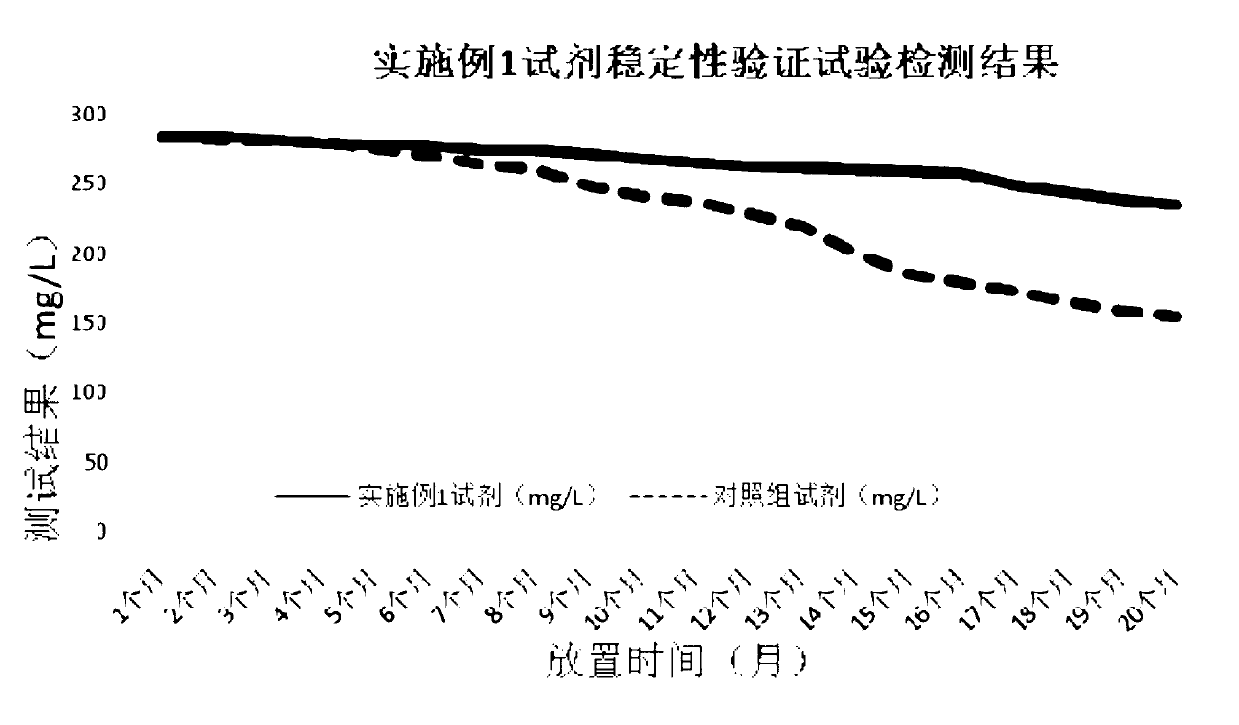
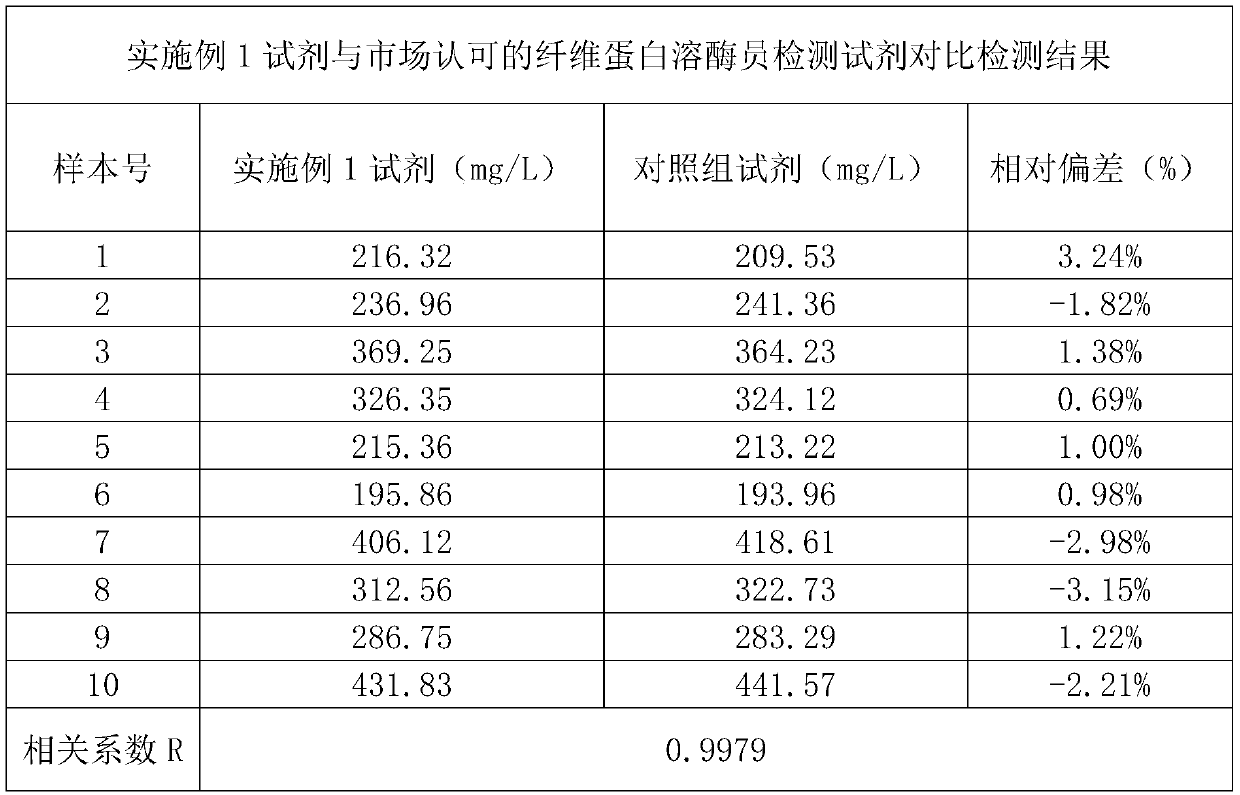
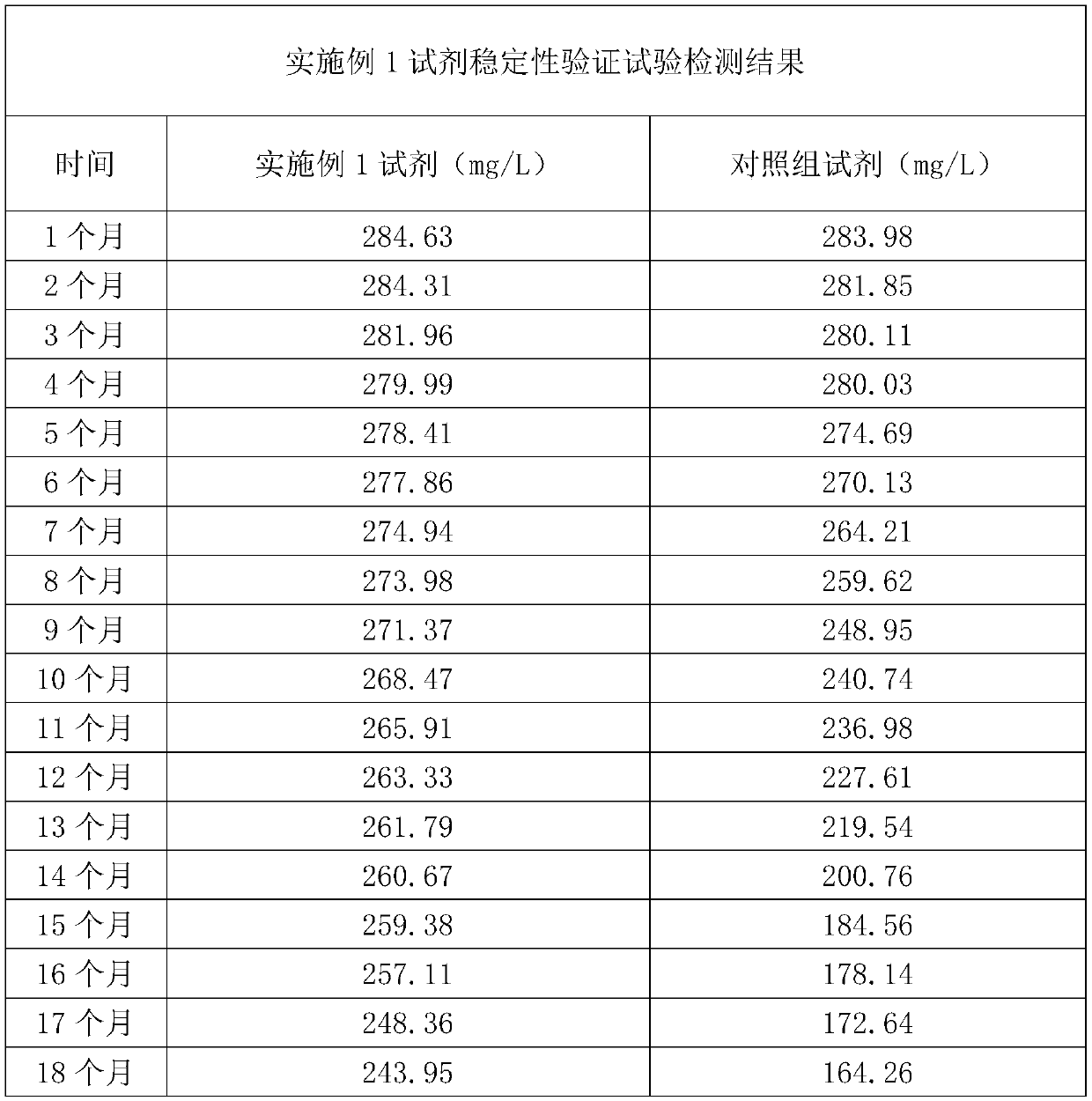
[0071] Accuracy verification test: the plasminogen detection reagent of embodiment 1 is used as an experimental group, and a kind of plasminogen detection reagent with good accuracy and good stability that has been recognized on the market is used as a control group for detection. Ten clinical serum samples were tested, and the test results are shown in Table 1. The results showed that the correlation coefficient of the two kits was 0.9979, indicating that the correlation between the two kits is relatively good. It proves that the added and changed components of the kit of the present invention will not affect its accuracy, and the kit still maintains a good accuracy.
[0072] Table 1 is the experimental result of the accuracy of reagents of the present invention and matched group reagents;
[0073]
Embodiment 3
[0075] Linear correlation verification test: Select a high-value sample with a plasminogen content of 420 mg / L, serially dilute it with normal saline, and prepare 7 samples with different concentrations, the concentrations are 420 mg / dL, 350 mg / dL, and 280 mg / dL. dL, 210mg / dL, 140mg / dL, 70mg / dL, 0mg / dL. The reagents of Example 1 and the control group were used for detection respectively. The samples of each concentration were measured three times, and the average values were taken respectively. The detection results are shown in Table 2.
[0076] Theoretical concentration (mg / L) Embodiment 1 reagent (mg / L) Control group reagent (mg / L) 0 -0.21 0.19 70 72.34 74.93 140 134.63 149.86 210 223.26 218.94 280 288.49 273.51 350 355.97 341.73 420 431.74 436.94 Correlation coefficient R 0.9995 0.9982
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com