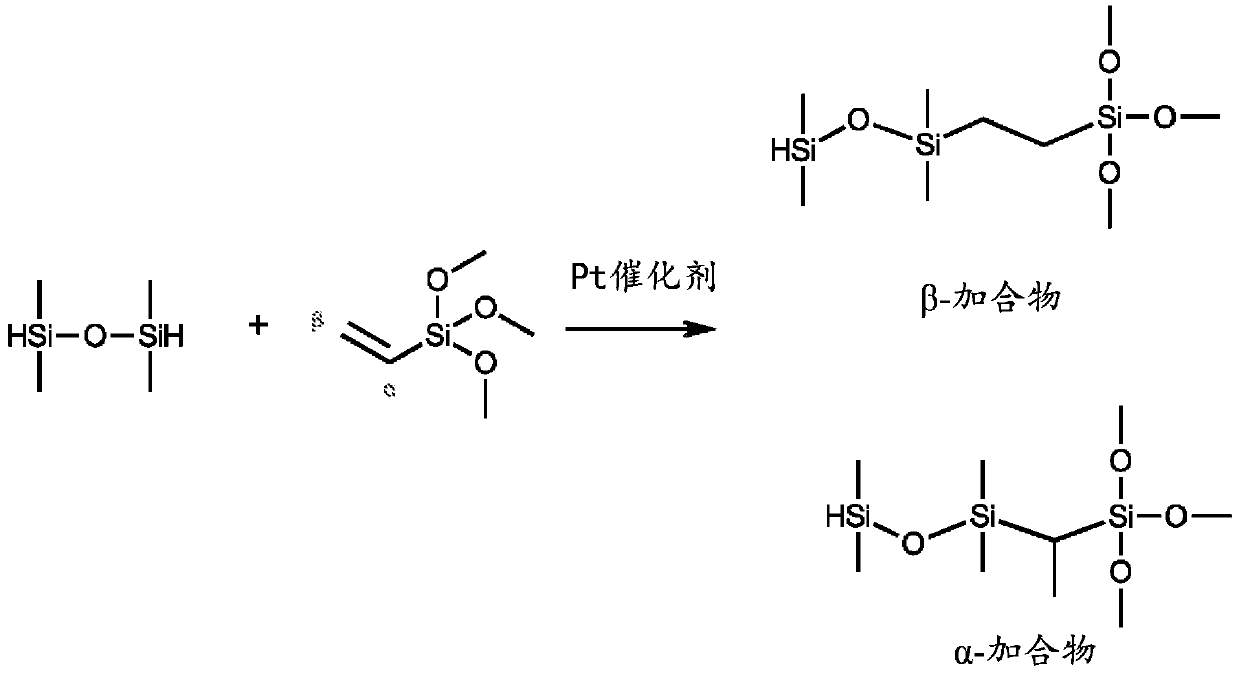
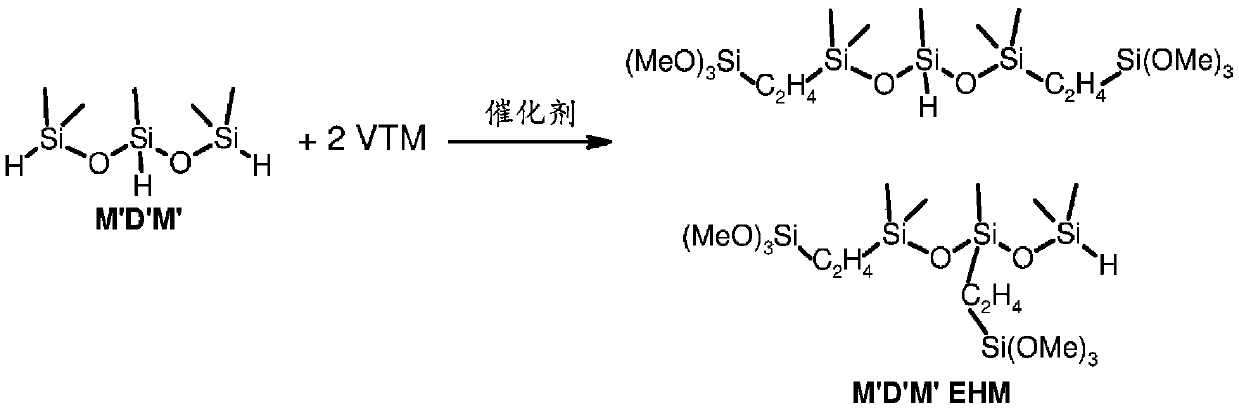
Method for hydrosilylation of aliphatically unsaturated alkoxysilanes and hydrogen terminated organosiloxane oligomers to prepare alkoxysilyl terminated polymers useful for functionalizing polyorganosiloxanes using rhodium catalyst
By using polyorganohydrogensiloxane oligomers, aliphatic unsaturated alkoxy groups in the hydrosilylation reaction of vinyltrimethoxysilane and 1,1,3,3-tetramethyldisiloxane The combination of silane and rhodium bisphosphine chelate solves the problems of low selectivity and many by-products, realizes an efficient method of preparing β-adducts, and improves process efficiency.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0126] A 250 mL round bottom flask including a stir bar was charged with 80 g of TMDS and an addition funnel containing 82.77 g of VTMS was secured to it. The apparatus was purged well with nitrogen and then preheated to 50°C. Start steady addition of VTMS (about 1 drop / sec) followed by 2.38 mL of 0.005M [Rh(dppe)Cl] 2 of THF solution. The addition was continued at such a rate that the temperature of the reaction mixture did not exceed 70°C after the addition of the first 50% of the VTMS, followed by the second half at 80°C. After the addition, the reaction mixture was allowed to react at 80 °C for 16 h. The reaction mixture was then purified by vacuum distillation to obtain a light fraction (solvent, unreacted reagents, and light by-products), a fraction of the desired product (distilled at approximately 1.25 torr (0.1666 kPa) and 55-60°C), and Heavy fraction in retort. The yield of the desired product fraction was 103.8 g (65% yield), and this fraction contained about 96...
Embodiment 2
[0127] Embodiment 2 (comparative example) :
[0128] In an air-tight glove box, a mixture of 1.1 g VTMS, 1 g TMDS, and 0.25 g of dodecane (internal standard) was added to a 20 mL scintillation vial including a stir bar. Then, add 30 μL of 0.01M Rh (PPh 3 ) 3 Cl in THF (heat the reagent solution to about 60° C. with stirring to dissolve the poorly soluble catalyst). After stirring at room temperature for 30 min, the reaction mixture was heated to 50 °C for 16 h. At this stage, an aliquot (approximately 150 μL) of the reaction mixture was withdrawn and injected into a GC vial and diluted with approximately 1 mL of xylene. Reactions were analyzed by GC-FID and GC-MS. Analysis indicated an overall yield of ETM of 62% with a linear:branched isomer selectivity ratio of 87:13. A small amount of unreacted starting material was observed. This Example 3 shows a lower selectivity to the desired β-adduct in the product compared to Example 1 . As will be shown in Example 20, this ...
Embodiment 3
[0129] Embodiment 3 (comparative example)—preparation of ethyltrimethoxysilyl-terminated tetramethyldisiloxane
[0130] In an air-tight glove box, a mixture of 1.1 g VTMS, 1 g TMDS, and 0.25 g of dodecane (internal standard) was added to a 20 mL scintillation vial including a stir bar. Then, 30 μL of a 0.01 MPt solution in THF in the form of Karstedt's catalyst (provided at 2% in xylene, Sigma Aldrich) was added (the reagent solution was heated to about 60° C. with stirring to dissolve the poorly soluble catalyst). After stirring at room temperature for 30 min, the reaction mixture was heated to 50 °C for 16 h. At this stage, an aliquot (approximately 150 μL) of the reaction mixture was withdrawn and injected into a GC vial and diluted with approximately 1 mL of xylene. Reactions were analyzed by GC-FID and GC-MS. Analysis indicated an overall yield of ETM product of 72% with a linear:branched isomer selectivity ratio of 64:36. A small amount of unreacted tetramethyldisil...
PUM
| Property | Measurement | Unit |
|---|---|---|
| rate of recovery | aaaaa | aaaaa |
| rate of recovery | aaaaa | aaaaa |
| time | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com