Vatalanib succinate intermediate and synthesis method and application thereof
A synthesis method and technology for reactants, applied in the field of medicine, can solve the problems of difficult control of high temperature solid-phase reaction conditions, unfavorable stirring, and excessive viscosity, and achieve the effects of good industrial prospects, short reaction time, and few reaction steps.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
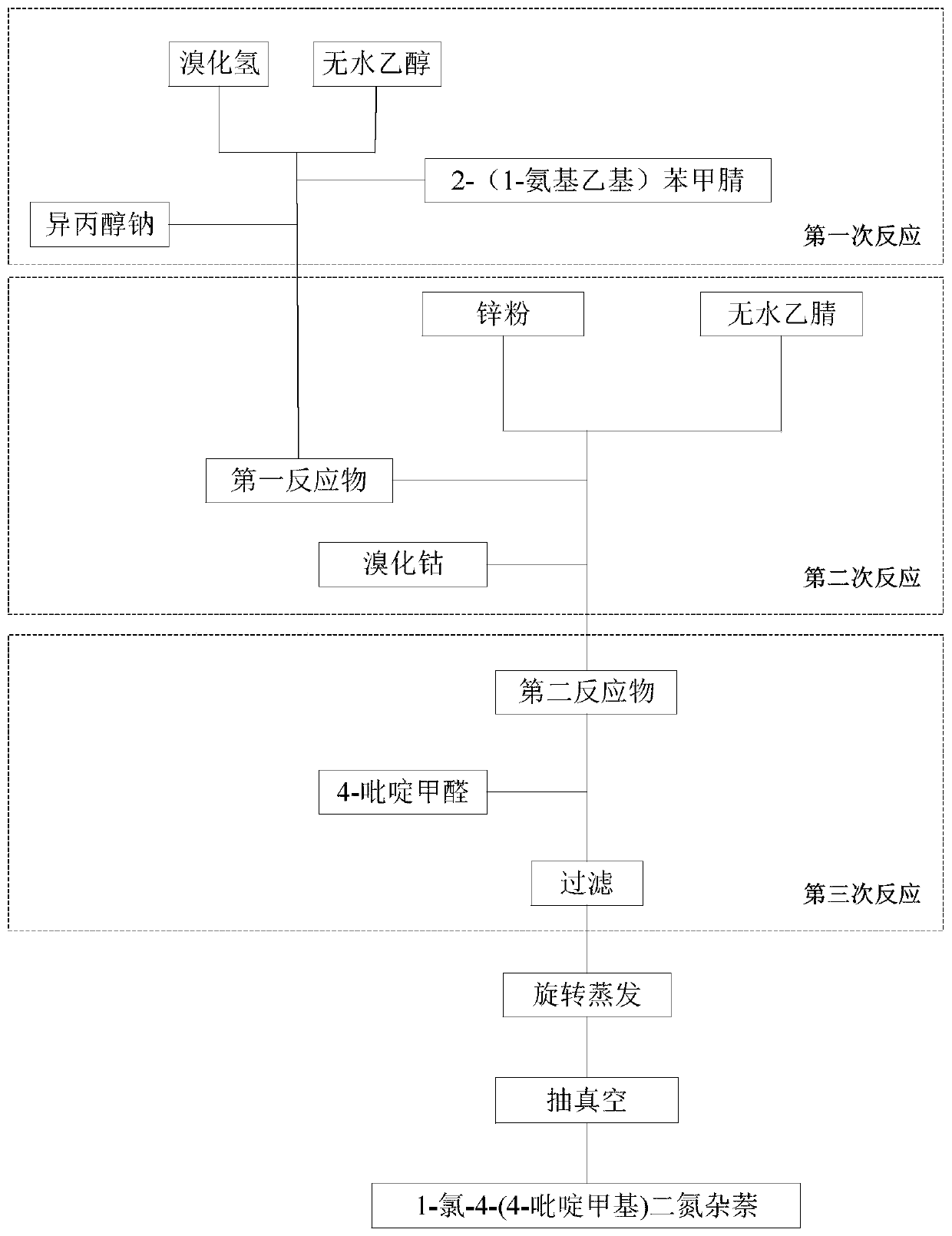
[0033] like figure 1 As shown, the present embodiment 1 provides a kind of synthetic method of 1-chloro-4-(4-pyridylmethyl)naphthalene, comprising the following steps: step S1, with 2-(1-aminoethyl)benzene Carbonitrile is used as the initial raw material to perform multiple reactions successively; and step S2, rotary evaporation and vacuum pumping to obtain the 1-chloro-4-(4-pyridylmethyl)phthalazine.
[0034] Specifically, the synthesis method of Example 1 selects 2-(1-aminoethyl)benzonitrile as the initial raw material, which is cheap and easy to obtain, avoids the use of lithium salts, avoids carbonization, and has fewer reaction steps, and the reaction The time is short, which is conducive to industrialized production. The chemical purity of the product can reach more than 90.582%, and the yield can reach more than 79.25%, which has good industrial prospects.
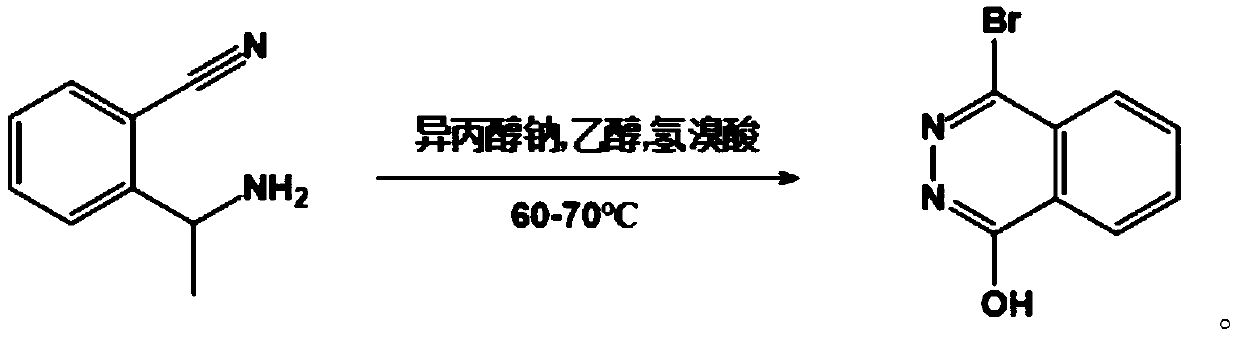
[0035] Further, the reaction formula of the first reaction is:
[0036]
[0037] As an optional implementat...
Embodiment 2
[0053] On the basis of Example 1, this Example 2 provides a 1-chloro-4-(4-pyridylmethyl)naphthalene, and the 1-chloro-4-(4-pyridylmethyl)bis The structural formula of aziridine is:
[0054]
[0055] Regarding the component content and specific implementation process of 1-chloro-4-(4-pyridyl)naphthyridine, please refer to the relevant discussion in Example 1, and will not repeat them here.
Embodiment 3
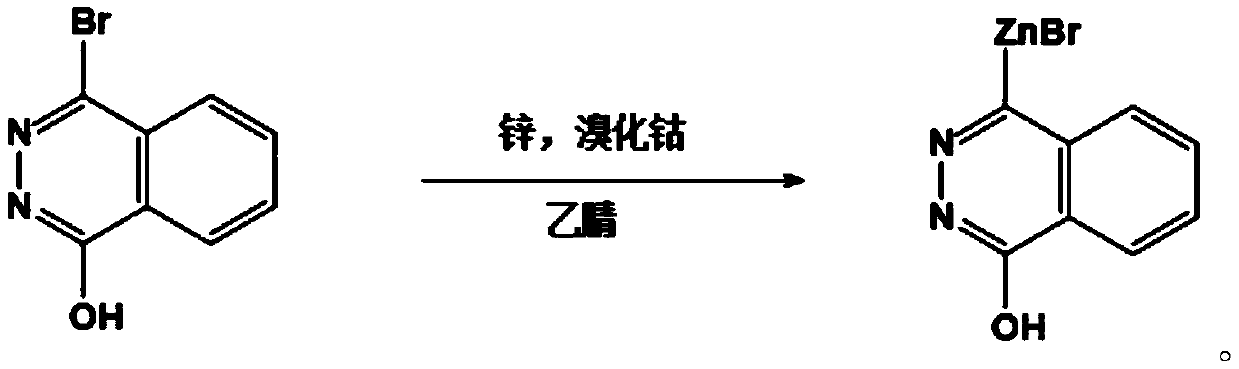
[0057] On the basis of Example 1, this Example 3 provides a first reactant for the synthesis of 1-chloro-4-(4-pyridylmethyl)naphthalene, the structural formula of the first reactant is:
[0058]
[0059] For the content of the components of the first reactant and the specific implementation process, please refer to the relevant discussion in Example 1, and details will not be repeated here.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com