Method for measuring dithionite by potassium ferricyanide differential spectrophotometry
A dithionite and spectrophotometry technology, applied in the measurement of color/spectral characteristics, etc., can solve the problem of large minimum detection limit, and achieve the effect of ensuring sensitivity and accuracy, accurate standard curve, and small error
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
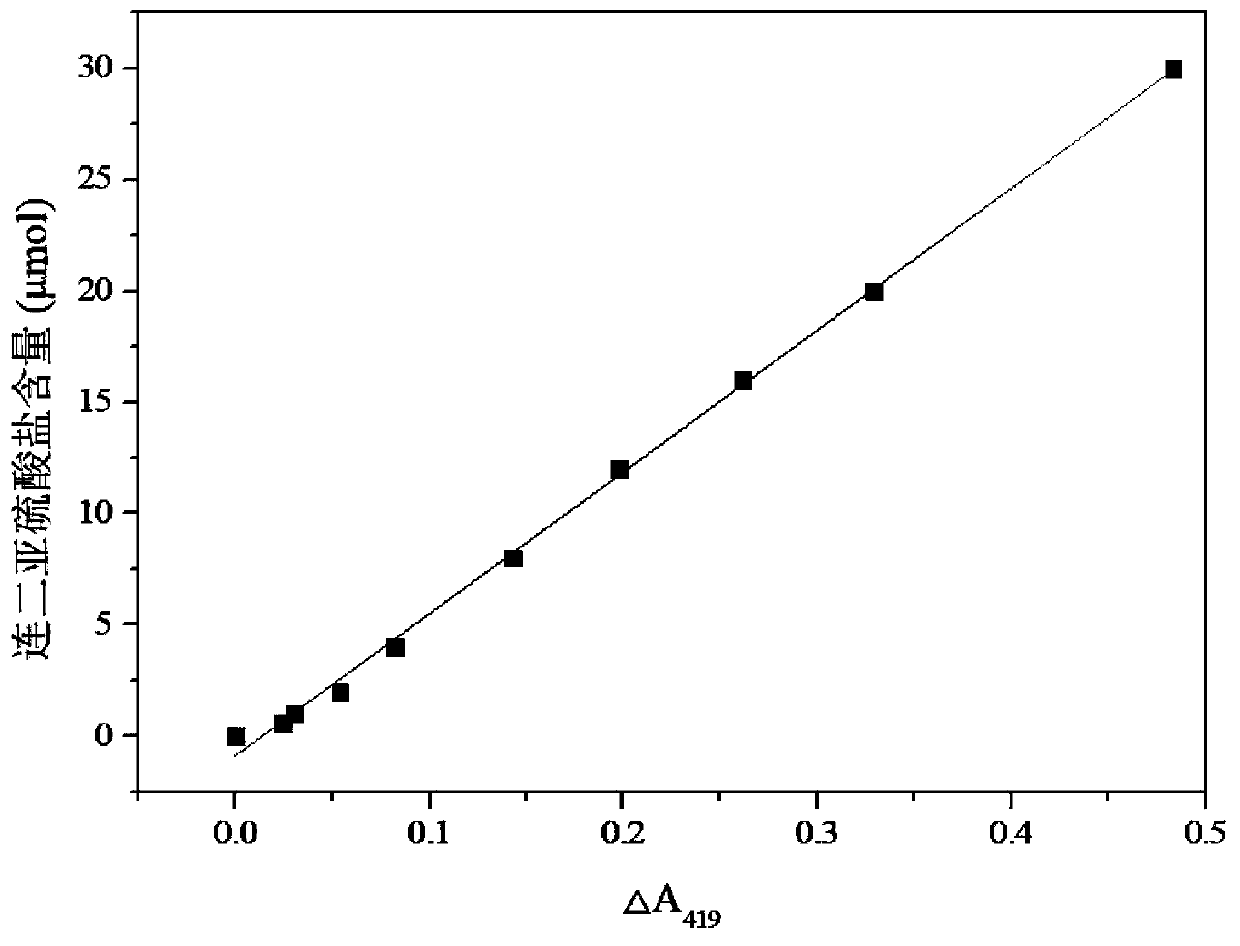
[0036] The present embodiment provides a method for measuring dithionite by potassium ferricyanide differential spectrophotometry, comprising the steps of:
[0037] S1: Add 5mL of sodium hydroxide solution with a concentration of 1mol / L into a 50mL stoppered colorimetric tube, and then continue to add 4mL of potassium ferricyanide solution with a concentration of 10mmol / L, and record it as mixed solution A 0 ; Finally, the mixture A was mixed with deionized water 0 Set the volume to 50mL, and record the absorbance at the characteristic absorption wavelength of 419nm as a 0 Add respectively 0.03, 0.05, 0.1, 0.2, 0.4, 0.6, 0.8, 1.0, 1.5mL concentration to 10 50mL colorimetric tubes with stoppers that mixed solution A is equipped with and be 20mmol / L sodium dithionite standard use solution, Dilute to the 50mL mark with deionized water, shake and shake well, react at 25°C for 40min, measure the absorbance value of the reaction mixture in each colorimetric tube at the characterist...
Embodiment 2
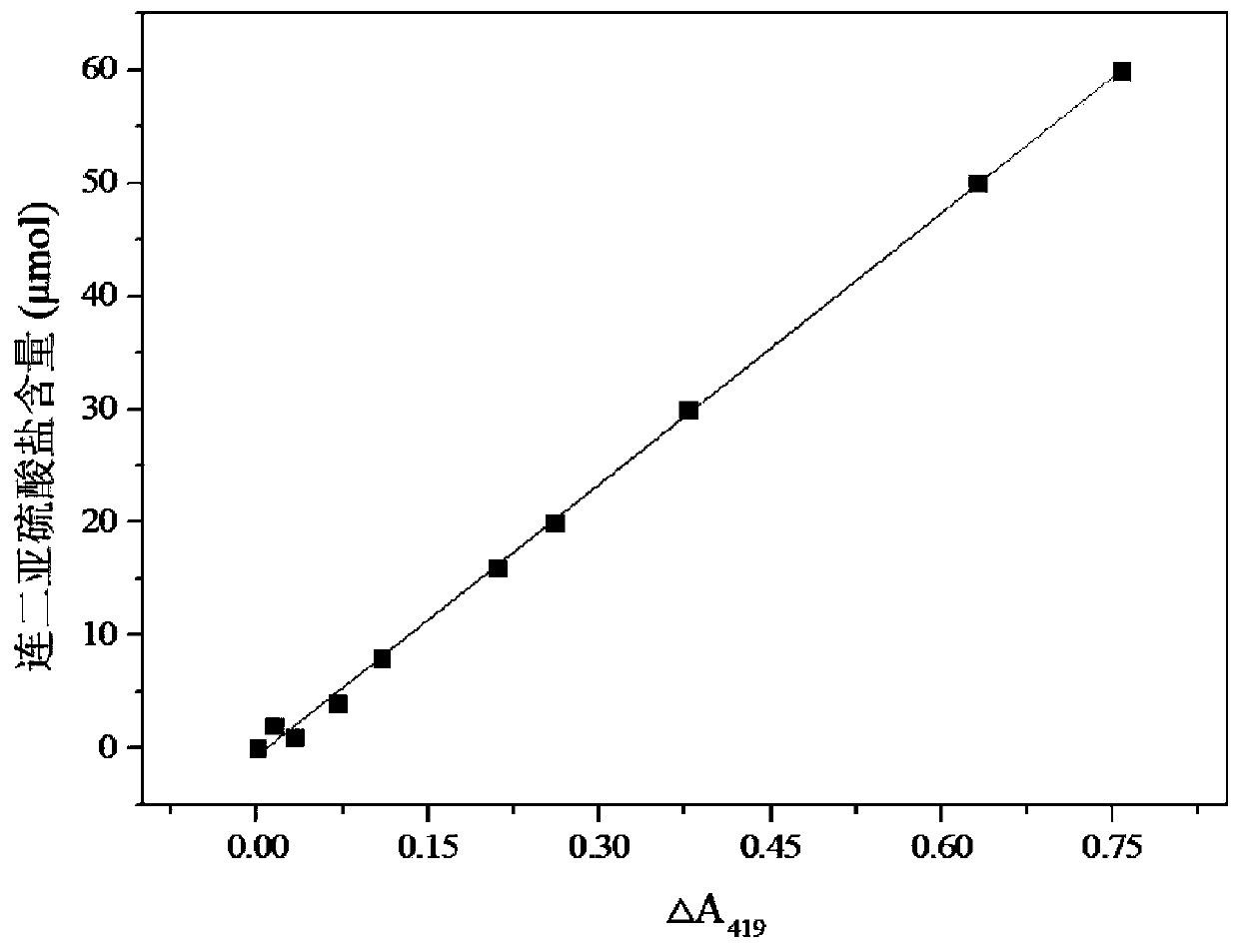
[0043] The present embodiment provides a method for measuring dithionite by potassium ferricyanide differential spectrophotometry, comprising the steps of:
[0044] S1: Add 5mL of potassium hydroxide solution with a concentration of 1mol / L into a 50mL stoppered colorimetric tube, and then continue to add 4mL of sodium ferricyanide solution with a concentration of 10mmol / L, and record it as mixed solution A 0 ; Finally, the mixture A was mixed with deionized water 0 Set the volume to 50mL, and record the absorbance at the characteristic absorption wavelength of 419nm as a 0 ; Add 0.05, 0.1, 0.2, 0.4, 0.6, 0.8, 1.0, 1.5, 2.5, 3.0mL of 20mmol / L magnesium dithionite to 10 50mL stoppered colorimetric tubes equipped with mixed solution A For the standard solution, dilute it to the 50mL mark with deionized water, shake it well, react at 50°C for 60min, and measure the absorbance value of the reaction mixture in each colorimetric tube at the characteristic absorption wavelength of 41...
Embodiment 3
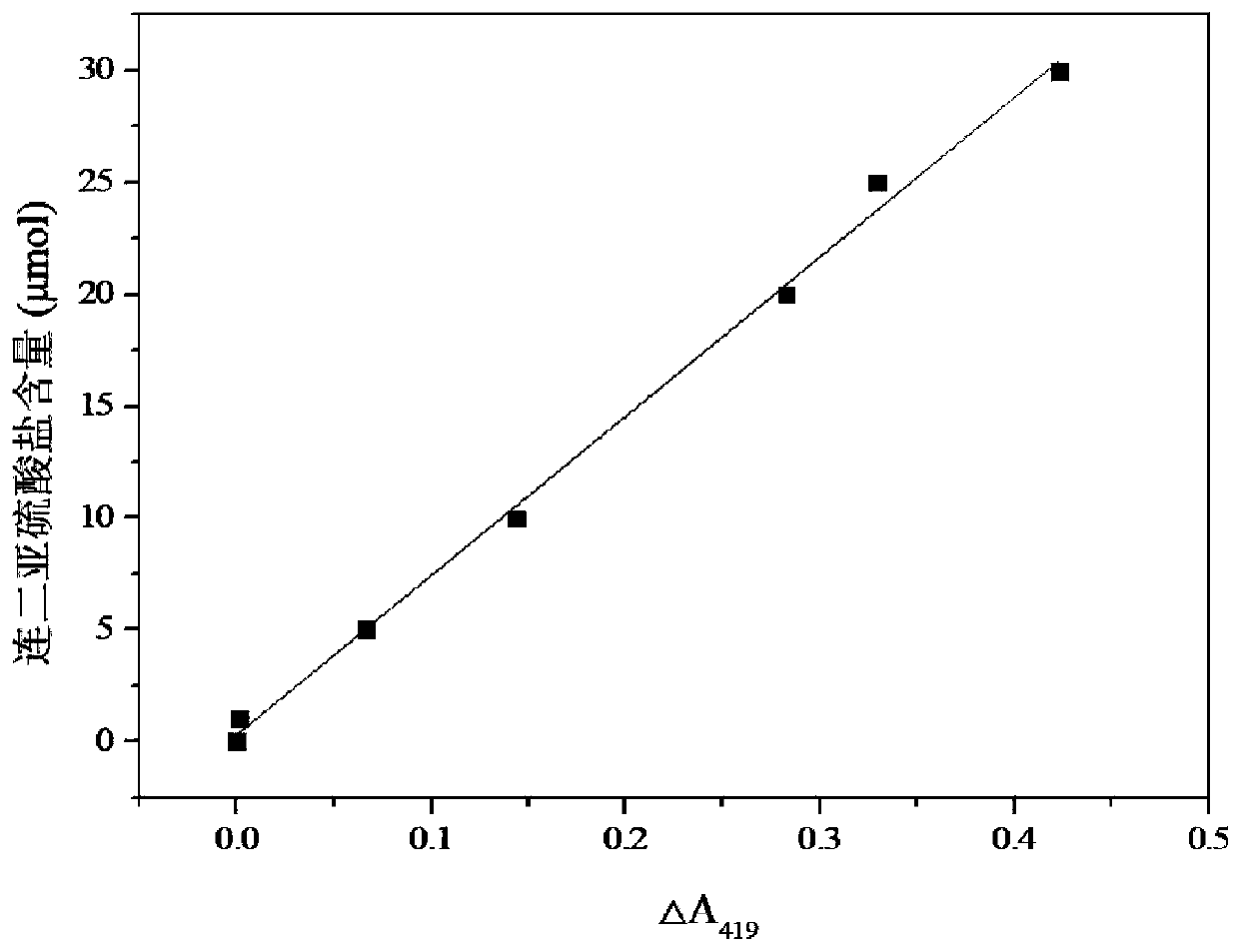
[0050] The present embodiment provides a method for measuring dithionite by potassium ferricyanide differential spectrophotometry, comprising the steps of:
[0051] S1: Add 1mL sodium hydroxide solution with a concentration of 1mol / L into a 50mL stoppered colorimetric tube, and then continue to add 1mL potassium ferricyanide solution with a concentration of 10mmol / L, and record it as mixed solution A 0 ; Finally, the mixture A was mixed with deionized water 0 Set the volume to 50mL, and record the absorbance at the characteristic absorption wavelength of 419nm as a 0 Add 0.05, 0.25, 0.5, 1.0, 1.25, 1.5mL standard solution of 20mmol / L sodium dithionite to 10 50mL stoppered colorimetric tubes equipped with mixed solution A, and dilute to volume with deionized water After reaching the 50mL marked line, shake and shake well, react at 25°C for 40min, measure the absorbance value of the reaction mixture in each colorimetric tube at the characteristic absorption wavelength of 419nm,...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com