Cholic acid derivative as well as preparation method and application thereof
A technology for cholic acids and derivatives is applied in the field of cholic acid derivatives and their preparation, and achieves broad application prospects, inhibits liver cell damage, and inhibits liver cell apoptosis.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
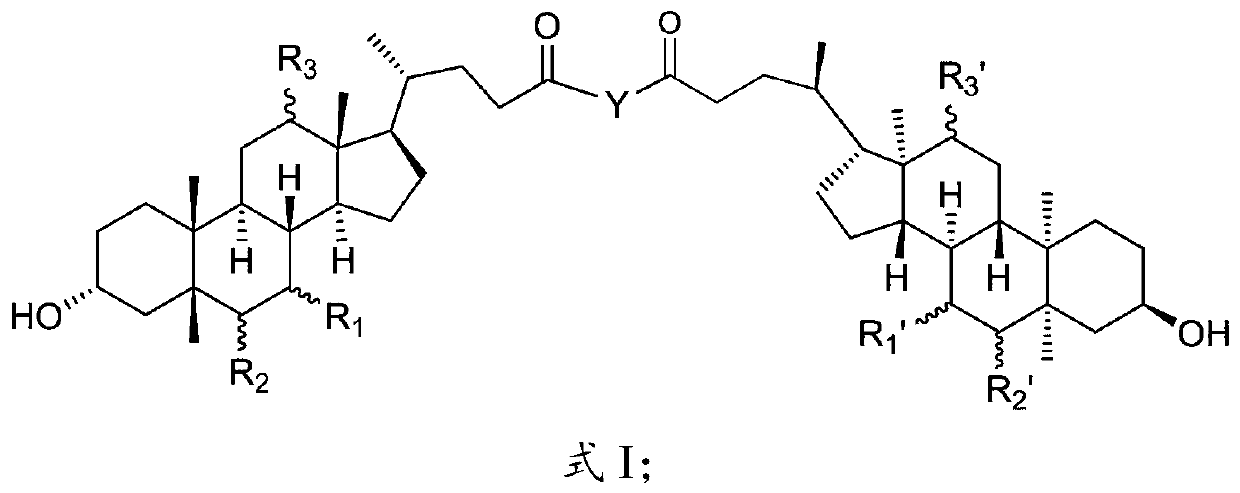
[0064] The present invention provides the preparation method of cholic acid derivative described in above-mentioned scheme, comprises the following steps:
[0065] When R 1 with R 1 ’ Same, R 2 with R 2 ’ Same, R 3 with R 3 ’ same, when Y is the following group,
[0066]
[0067]
[0068] The preparation method of described cholic acid derivatives comprises the following steps:
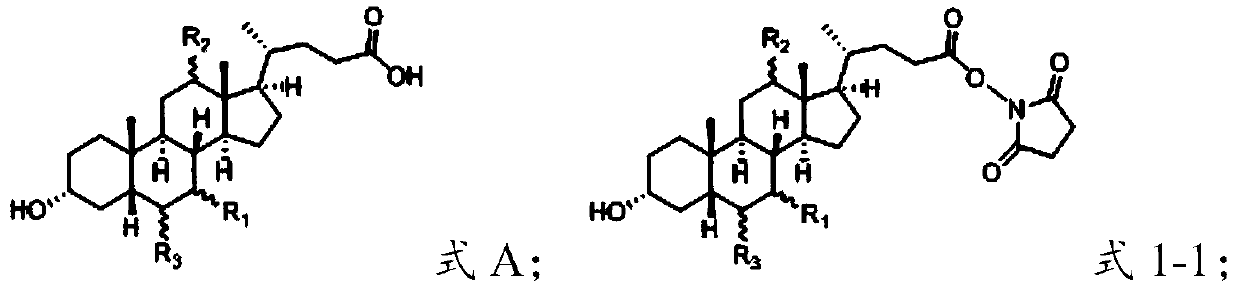
[0069] (1) under the action of a condensing agent, the compound of the structure shown in formula A is reacted with N-hydroxysuccinimide to obtain an intermediate having the structure shown in formula 1-1;
[0070]
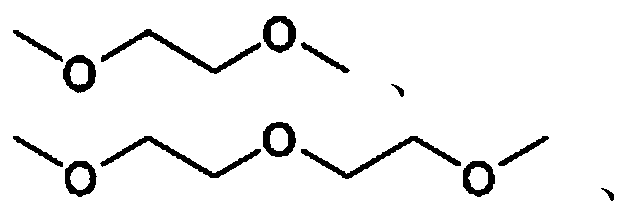
[0071] (2) The intermediate having the structure shown in formula 1-1, the compound Y1 and the solvent are mixed and reacted to obtain cholic acid derivatives; the compound Y1 has one of the following structures:
[0072]
[0073] Concrete synthetic route is as shown in formula one:
[0074]
[0075] In the present invention, under the action of a condensing agent, ...
Embodiment 1
[0127](4R,4'R)-N,N'-(ethane-1,2-diyl)bis(4-((3R,5S,7S,8R,9S,10S,13R,14S,17R)-3 , 7-dihydroxyl-10,13-dimethylhexadecahydro-1H-cyclopenta[a]phenanthrene-17-yl) pentamide) synthesis; denoted as compound 1a, the steps are as follows:
[0128] Add ursodeoxycholic acid (10g, 25.47mmol), EDCI (4.40g, 38mmol), N-hydroxysuccinimide (3.5g, 30.5mmol) and anhydrous DMF (50mL) to the flask successively, at 35°C , Stir the reaction under the protection of argon for 12h. Water (120 mL) was added to the reaction and extracted with ethyl acetate (3 x 50 mL). The combined organic phases were washed with saturated NaHCO 3 solution (3×50mL) and washed with Na 2 SO 4 dry. The extract was concentrated under reduced pressure to obtain the product (1-1) (8.08 g, 64%) as a white powder. 1 HNMR (600MHz, CD 3 OD-d 4 )δ3.55-3.46(m,2H),2.84(s,4H),2.72-2.64(m,1H),2.62-2.52(m,1H),2.07(dt,J=12.8,3.4Hz,1H) ,1.98-1.79(m,4H),1.68-1.18(m,16H),1.13(q,J=9.5Hz,1H),1.09-1.04(m,1H),1.03-0.98(m,6H),0.76 –0.7...
Embodiment 2
[0132] Ethane-1,2-diyl(4R,4'R)-bis(4-(((3R,5S,7S,8R,9S,10S,13R,14S,17R)-3,7-dihydroxy- 10,13-hexadecyldimethyl-1H-cyclopenta[a]phenanthrenyl-17-yl) pentanoate) synthesis, denoted as compound 2a; The steps are as follows:
[0133] Add ursodeoxycholic acid (3g, 7.64mmol), 1,4-dioxane (15mL), 3,4-dihydro-2H-pyran (1.93g, 22.9mmol) and p-toluenesulfonate to the flask successively Acid hydrate (0.29g, 1.53mmol), stirred at room temperature for 3h. Water (50 mL) was added to the reaction and extracted with ethyl acetate (3 x 30 mL). The combined extracts were washed with anhydrous Na 2 SO 4 Dry, concentrate under reduced pressure, and purify by silica gel column chromatography. Elution with 1:10 to 1:5 ethyl acetate-n-hexane gave (2-1) (2.8 g, 65%) as a white powder. 1 H NMR (600MHz, Chloroform-d) δ3.94–3.82(m,3H),3.64–3.32(m,3H),2.43–2.34(m,1H),2.29–2.20(m,1H),2.01–0.96 (m,22H),0.95–0.91(m,6H),0.69–0.63(m,3H).
[0134] Put (2-1) (0.5g, 0.9mmol), HOBT (0.15g, 1.08mmol), EDCI ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com