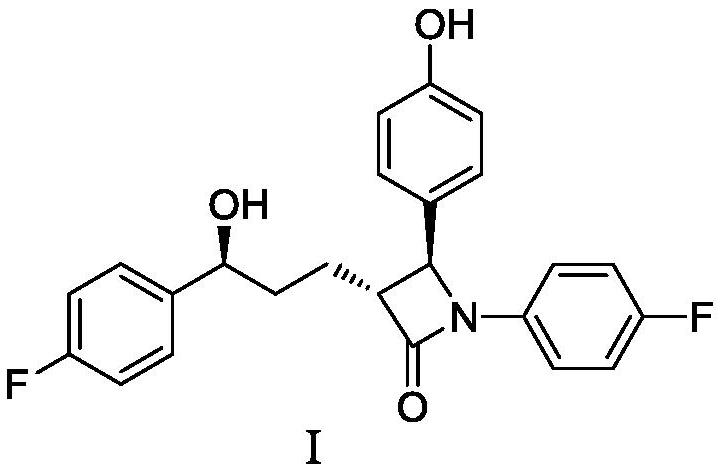
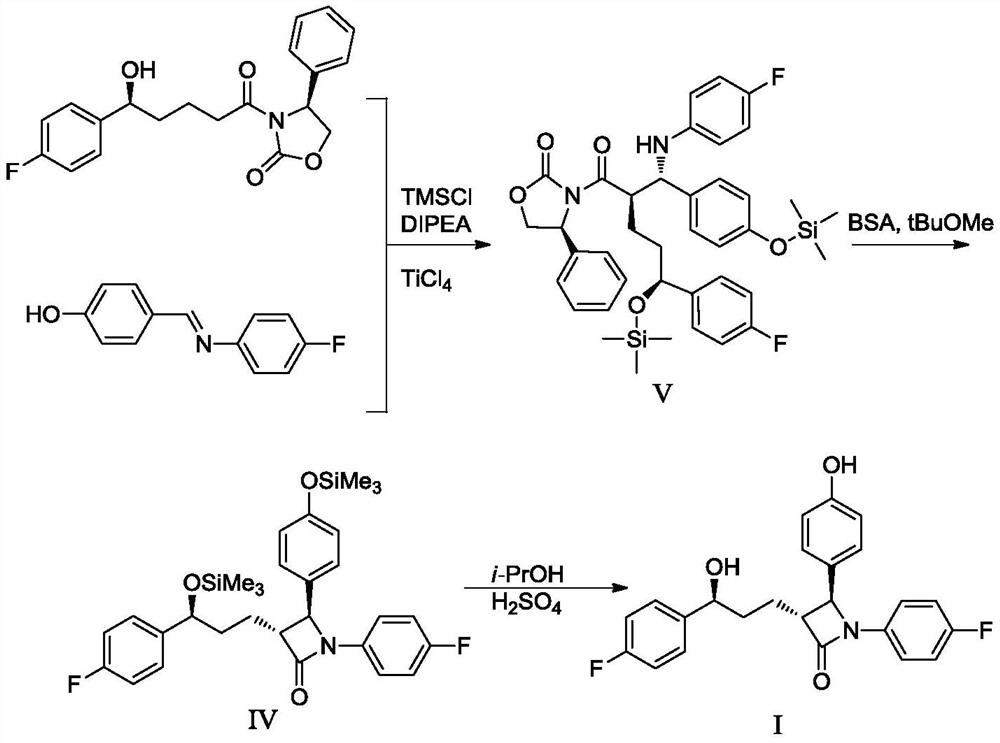
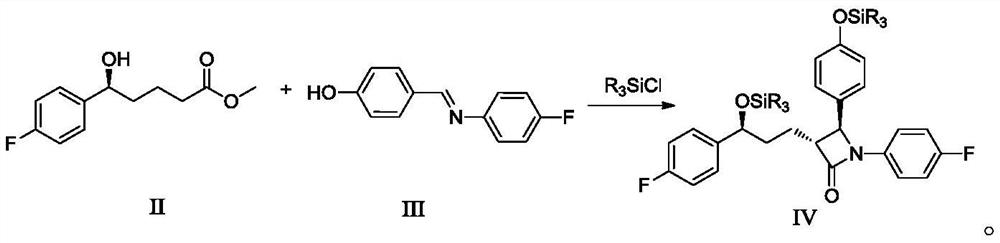
Preparation method of ezetimibe and its intermediate
A technology for ezetimibe and intermediates, which is applied in the field of preparation of ezetimibe and its intermediates, can solve the problems of unsuitability for industrial production, poor atom economy, poor product purity, etc., and avoid connecting chiral groups substrate, high total yield, and low production cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0048] Example 1: Preparation of ezetimibe intermediate IV (R=methyl)
[0049]
[0050] Under nitrogen protection, add 23.0 g (0.102 mol) of ezetimibe intermediate II (5S-5-(4-fluorophenyl)-5-hydroxyvaleric acid methyl ester) and ezetimibe intermediate to 345 mL of tetrahydrofuran Body III (4-[[(4-fluorophenyl)imine]methyl]-phenol) 32.9g (0.153mol), cooled to -10 ℃ ~ 0 ℃, added diisopropylethylamine 39.5g ( 0.306 mol) and 29.9 g (0.275 mol) of trimethylchlorosilane, and stirred at -10°C to 0°C for 2 hours to 3 hours. Add (+)-N-benzyl-(3R,4R)-bis(diphenylphosphine)pyrrolidine 0.54g (0.00102mol), and then slowly add 2.0mol / L lithium diisopropylamide tetrahydrofuran / ethylbenzene / heptane Alkanes solution 102mL (0.204mol), stirred at -10°C to 0°C for 1 hour to 2 hours. Adding mass concentration is 5% ammonium chloride aqueous solution (the described mass concentration refers to the quality of ammonium chloride accounts for the percentage of ammonium chloride aqueous solution t...
Embodiment 2
[0051] Example 2: Preparation of Ezetimibe Intermediate IV (R=Ethyl)
[0052]
[0053] Under nitrogen protection, 23.0 g (0.102 mol) of ezetimibe intermediate II (5S-5-(4-fluorophenyl)-5-hydroxyvaleric acid methyl ester) was added to 230 mL of 2-methyltetrahydrofuran, and Zemibe intermediate III (4-[[(4-fluorophenyl)imine]methyl]-phenol) 26.4g (0.123mol), cooled to 0 ℃ ~ 10 ℃, added 49.2g of tri-n-butylamine ( 0.265mol) and 36.9g (0.245mol) of triethylchlorosilane, stirred at 0°C to 10°C for 1 hour to 2 hours. Add (+)-N-benzyl-(3R,4R)-bis(diphenylphosphine)pyrrolidine 0.27g (0.00051mol), and then slowly add 2.0mol / L lithium diisopropylamide tetrahydrofuran / ethylbenzene / heptane Add 77 mL (0.154 mol) of alkane solution, and stir at 0°C to 10°C for 2 hours to 3 hours. Adding mass concentration is 5% ammonium chloride aqueous solution (the described mass concentration refers to the quality of ammonium chloride accounts for the percentage of ammonium chloride aqueous solution ...
Embodiment 3
[0054] Example 3: Preparation of Ezetimibe Intermediate IV (R=Propyl)
[0055]
[0056] Under nitrogen protection, 23.0 g (0.102 mol ), ezetimibe intermediate III (4-[[(4-fluorophenyl)imine]methyl]-phenol) 39.5g (0.183mol), cooled to -20℃~-10℃, added triethyl 35.0 g (0.346 mol) of amine and 60.9 g (0.316 mol) of tripropylchlorosilane were stirred at -20°C to -10°C for 3 hours to 4 hours. Add (+)-N-benzyl-(3R,4R)-bis(diphenylphosphine)pyrrolidine 1.08g (0.00204mol), and then slowly add 2.0mol / L lithium diisopropylamide tetrahydrofuran / ethylbenzene / heptane Add 102 mL (0.204 mol) of alkane solution, and stir at -20°C to -10°C for 3 hours to 4 hours. Adding mass concentration is 5% ammonium chloride aqueous solution (the described mass concentration refers to the quality of ammonium chloride accounts for the percentage of ammonium chloride aqueous solution total mass) 200mL, vacuum concentration removes most of organic solvent (35 ℃~45 ℃, -0.085MPa~-0.095MPa), extracted thre...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com