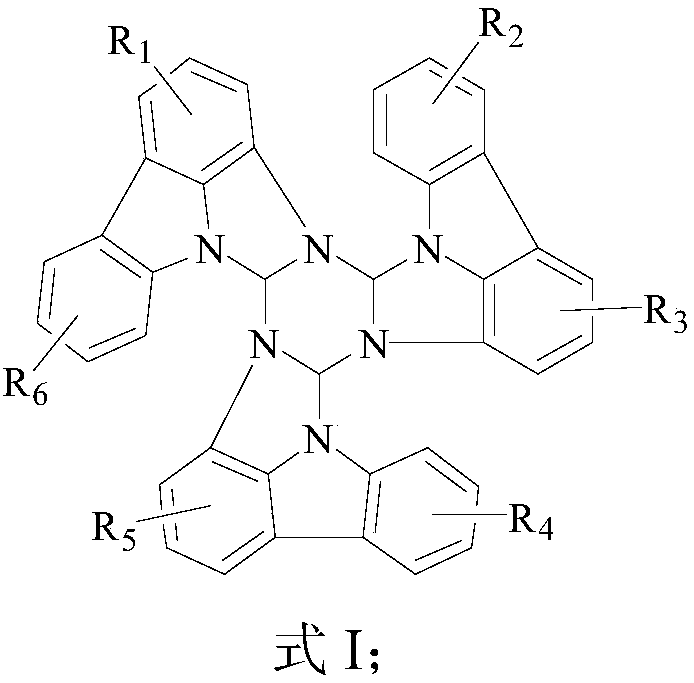
Organic electroluminescent material, a preparation method and applications thereof
An electroluminescent material and luminescent technology, applied in the direction of luminescent materials, organic chemistry, chemical instruments and methods, etc., can solve problems such as lifespan, efficiency, power consumption, and thermal stability unsatisfactory
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
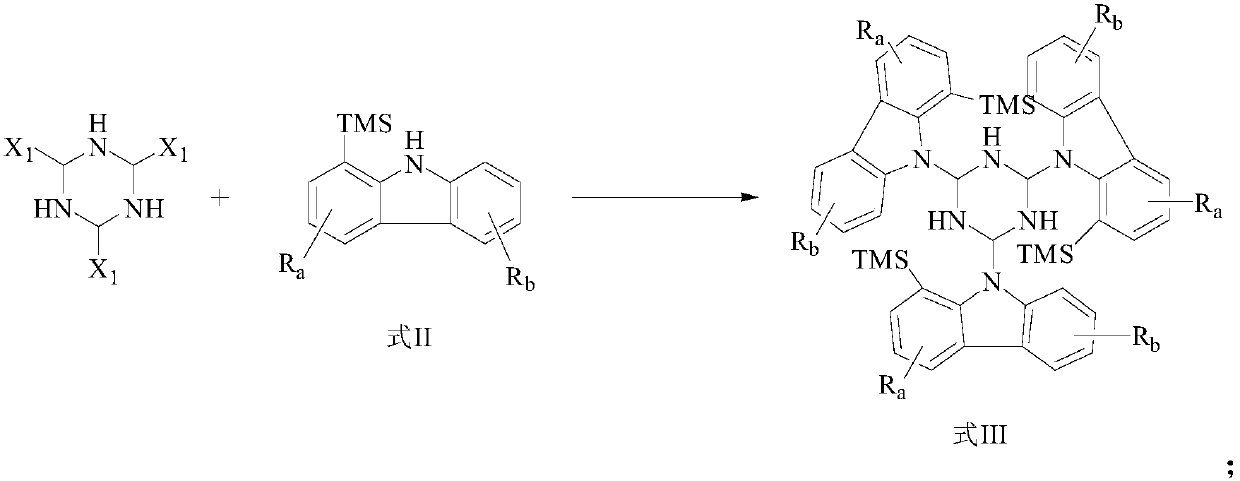
[0127] In this example, compound 1 was prepared by the following preparation method, and the reaction scheme is as follows:
[0128]
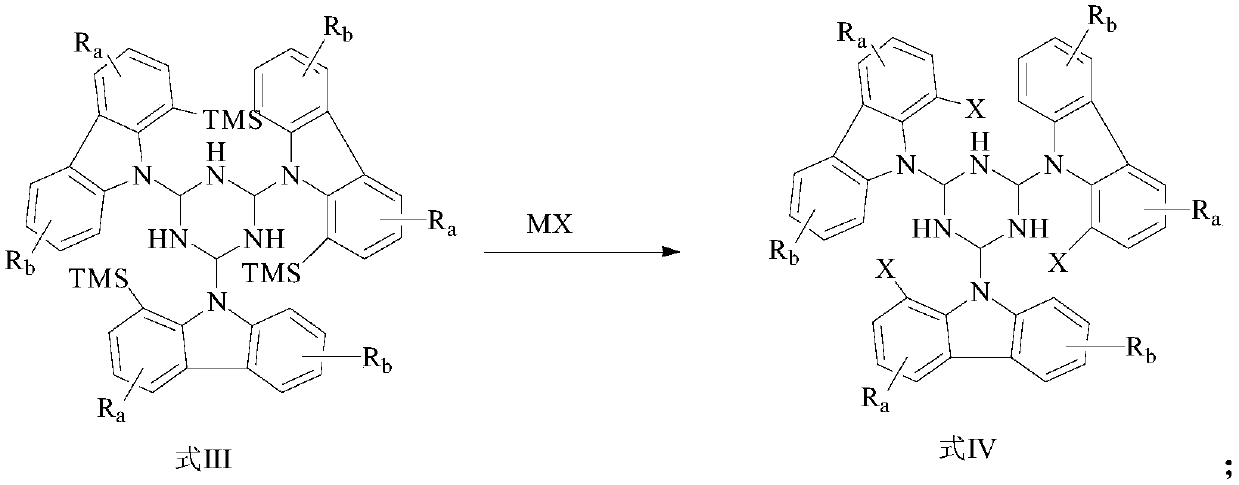
[0129] The specific preparation method and conditions are as follows: 4.5g of 8-trimethylsilane-7H-benzocarbazole, 5g of anhydrous cesium carbonate powder, 100mL of anhydrous 1,4-dioxane and 0.2g of Pd 2 dba 3 Add it to a 200mL three-neck bottle, vacuumize and replenish nitrogen for 30min. Keeping at 101° C., 2 g of 2,4,6-tribromo-1,3,5-hydrotriazine was added dropwise, and the reaction was refluxed in the dark for 24 hours. KBr was added dropwise, and the plate was tracked until the reaction was complete. The device was cooled to room temperature, crystallized by adding a large amount of water, and filtered with suction to obtain 4.2 g of solid, which was intermediate 1. 4.2 g of Intermediate 1, 5 g of anhydrous cesium carbonate powder, 100 mL of anhydrous 1,4-dioxane and 0.2 g of Pd 2 dba 3 Add it to a 200mL three-necked flask, under ...
Embodiment 2
[0132] In this example, compound 2 was prepared, and the preparation method was similar to that of compound 1, and the specific reaction process was as follows:
[0133]
[0134] Its preparation process is similar to the preparation of Example 1 Compound 1, except that the raw material of 8-trimethylsilane-7H-benzocarbazole is replaced by The yield of compound 2 was prepared in 44%.
[0135] Characterization data of compound 2: Tg(DSC) 120°C, purity 99.9%, 1 H NMR (400MHz, DMSO) δ7.32(s,3H),7.17(d,3H),7.16(d,3H),6.87(m,3H),6.76(d,3H),6.44(d,3H) ,5.96(s,3H),1.34(s,9H).
Embodiment 3
[0137] In this example, compound 3 was prepared, and the preparation reaction flow was as follows:
[0138]
[0139] The specific preparation method and conditions are as follows: 1.5g reactant 1, 4g anhydrous cesium carbonate powder, 100mL anhydrous 1,4-dioxane and 0.15g Pd 2 dba 3 Add it to a 200mL three-neck bottle, vacuumize and replenish nitrogen for 30min. Keeping at 101°C, 2g of 2-bromo-4,6trimethylsilane-hydrotriazine was added dropwise, and the reaction was refluxed in the dark for 24 hours. KBr was added dropwise, and the plate was tracked until the reaction was complete. The device was cooled to room temperature, and crystallized by adding a large amount of water, and suction filtered to obtain 2.8 g of solid, which was intermediate 2. 2.8 g of intermediate 2, 5 g of anhydrous cesium carbonate powder, 100 mL of anhydrous 1,4-dioxane and 0.2 g of Pd 2 dba 3 Put it into a 200mL three-necked flask, under nitrogen atmosphere, add 8-trimethylsilanecarbazole dropw...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Thickness | aaaaa | aaaaa |
| Thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com