A high-efficiency organic solar cell based on fluorinated electron donor and fluorinated electron acceptor
An organic solar cell and electron donor technology, which is applied in the field of solar cells, can solve the problems of the change of the morphology of the active layer, the drop of the open circuit voltage of the battery, and the small electron mobility, and achieves the enhancement of charge transfer, large short-circuit current density, and electron mobility. The effect of increased mobility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
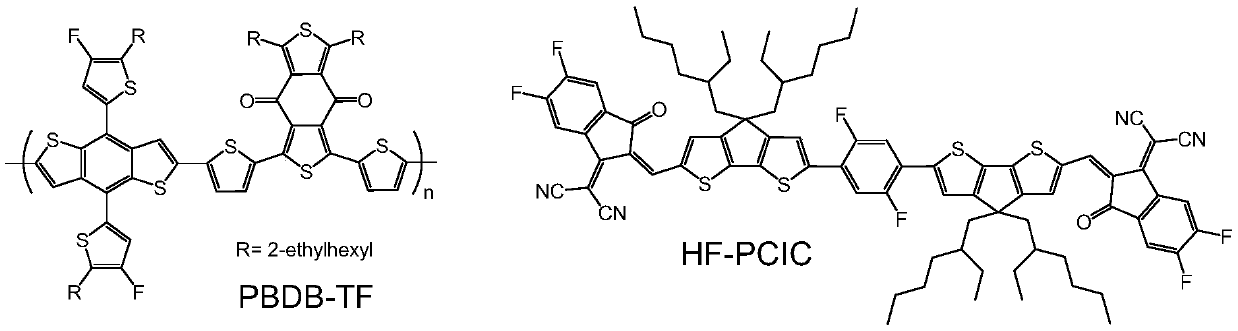
[0018] HF-PCIC is prepared by a simple Knoevenagel reaction using DF-PC-CHO as a raw material. The specific synthetic route is as follows:
[0019]
[0020] Among them, DF-PC-CHO is synthesized according to the method disclosed in Chinese patent 201710409496.1, and the specific synthesis steps of HF-PCIC are:
[0021] Add 0.3g of raw material DF-PC-CHO, 0.35g of fluorocyanindanone (IC-2F) and 50mL of dried chloroform into a two-necked round-bottomed flask, freeze the resulting mixed solution with liquid nitrogen, and then vacuumize it three times Nitrogen filled cycle. After thawing, 0.6 mL of pyridine was added under nitrogen atmosphere, and the resulting mixture was heated to reflux at 65°C for 12 hours. After the reaction was completed, the solvent was removed by rotary evaporation, and the crude product obtained was purified with a silica gel column using a n-hexane / dichloromethane mixture (1:3) as the eluent to obtain 0.27g of the final product HF-PCIC (dark brown sol...
Embodiment 2
[0024] The transparent conductive glass with strip-shaped ITO (cathode) etched on the surface is cleaned with cleaning agent, deionized water, acetone and isopropanol by ultrasonic oscillation, dried, and then treated with ultraviolet ozone for 15 minutes; then on the surface of the conductive glass A layer of ZnO was spin-coated at 3500 rpm for 60 seconds, and then annealed at 170° C. for 20 minutes. Then the sheet is transferred to the glove box, and the weight ratio of PBDB-TF:HF-PCIC (PBDB-TF can be prepared according to the document Advanced Materials, 2015, 27, 4655) is 1:1, and the total concentration is 20 mg / mL of chlorobenzene The mixed solution was spin-coated at a speed of 2000 rpm for 60 seconds to obtain an active layer with a thickness of 100 nm. Finally, a layer of MoO with a thickness of 10 nm was evaporated with an evaporation apparatus. 3 interface layer and a 100nm thick Ag electrode (anode), resulting in an effective area of 6mm 2 organic solar cells. ...
Embodiment 3
[0027]The transparent conductive glass with strip-shaped ITO (cathode) etched on the surface is cleaned with cleaning agent, deionized water, acetone and isopropanol by ultrasonic oscillation, dried, and then treated with ultraviolet ozone for 15 minutes; A layer of ZnO was spin-coated at 3500 rpm for 60 seconds, and then annealed at 170° C. for 20 minutes. Then the sheet was transferred to the glove box, and the chlorobenzene mixture with a weight ratio of PBDB-TF:HF-PCIC of 1:1.2 and a total concentration of 20mg / mL was spin-coated for 60 seconds at a speed of 2000rpm to obtain a layer of 100nm thick active layer. Finally, a layer of MoO with a thickness of 10 nm was evaporated with an evaporation apparatus. 3 interface layer and a 100nm thick Ag electrode (anode), resulting in an effective area of 6mm 2 organic solar cells.
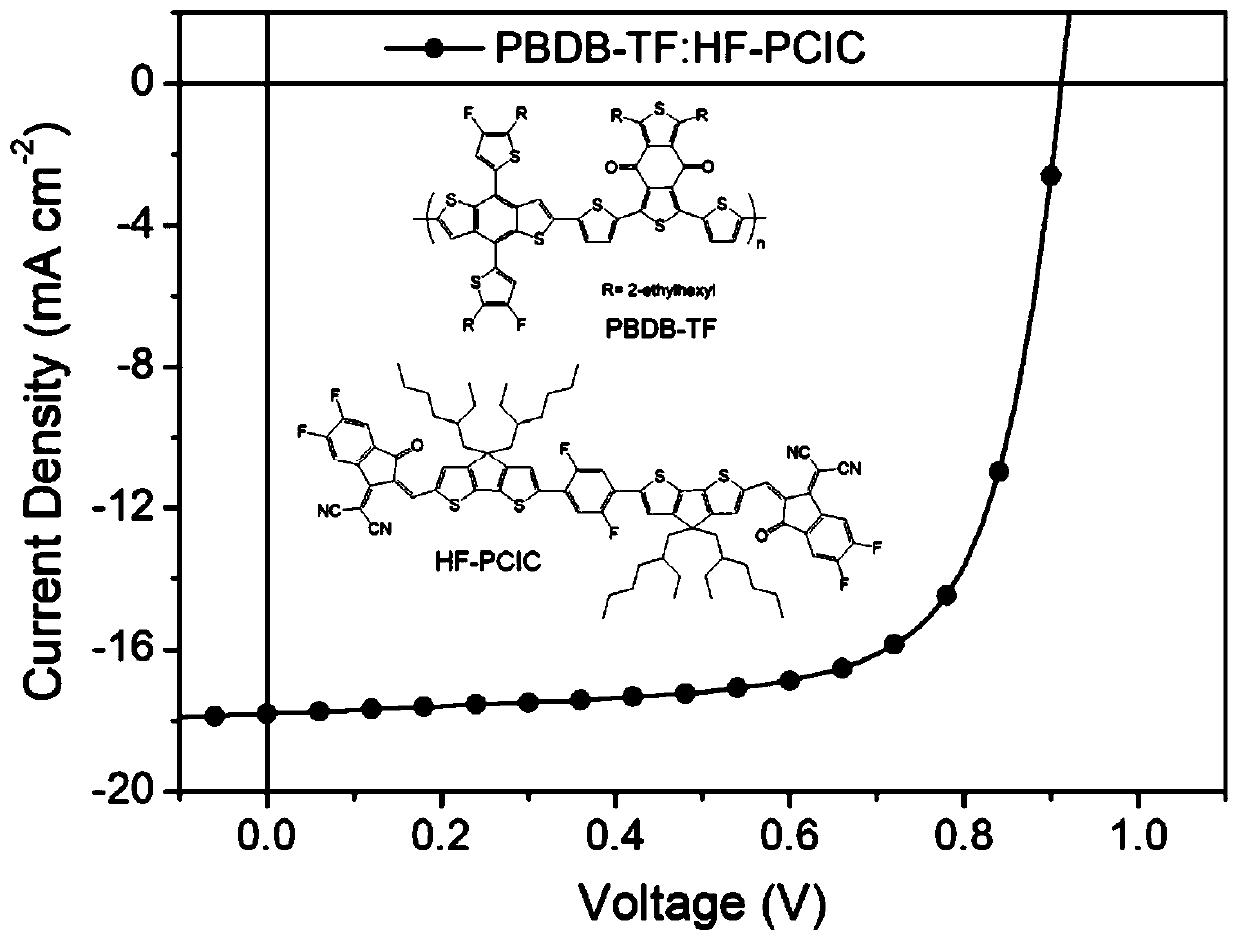
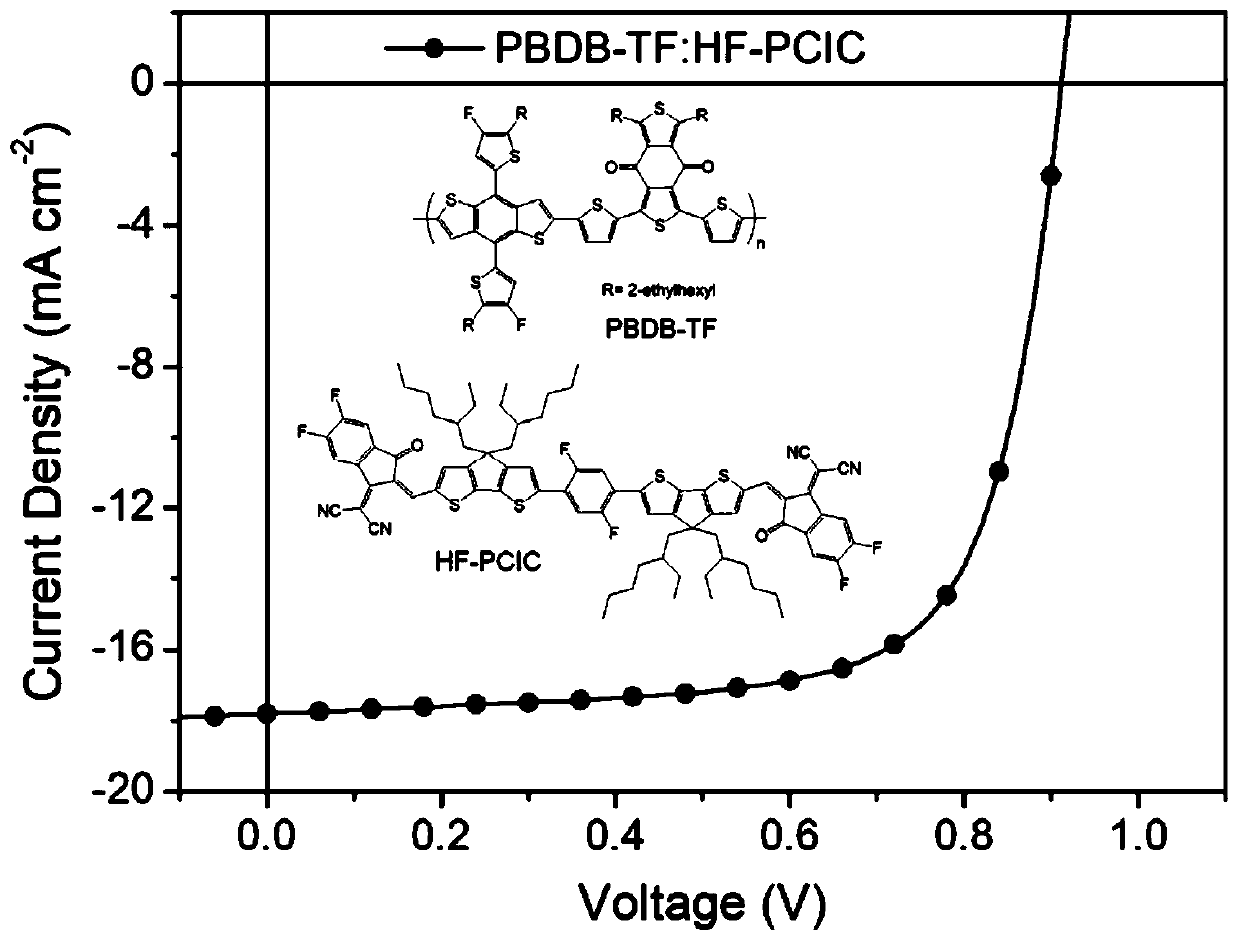
[0028] When the light intensity is 100mW / cm 2 The current-voltage curve of the device is tested under the simulated sunlight of AM1.5, and the o...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com