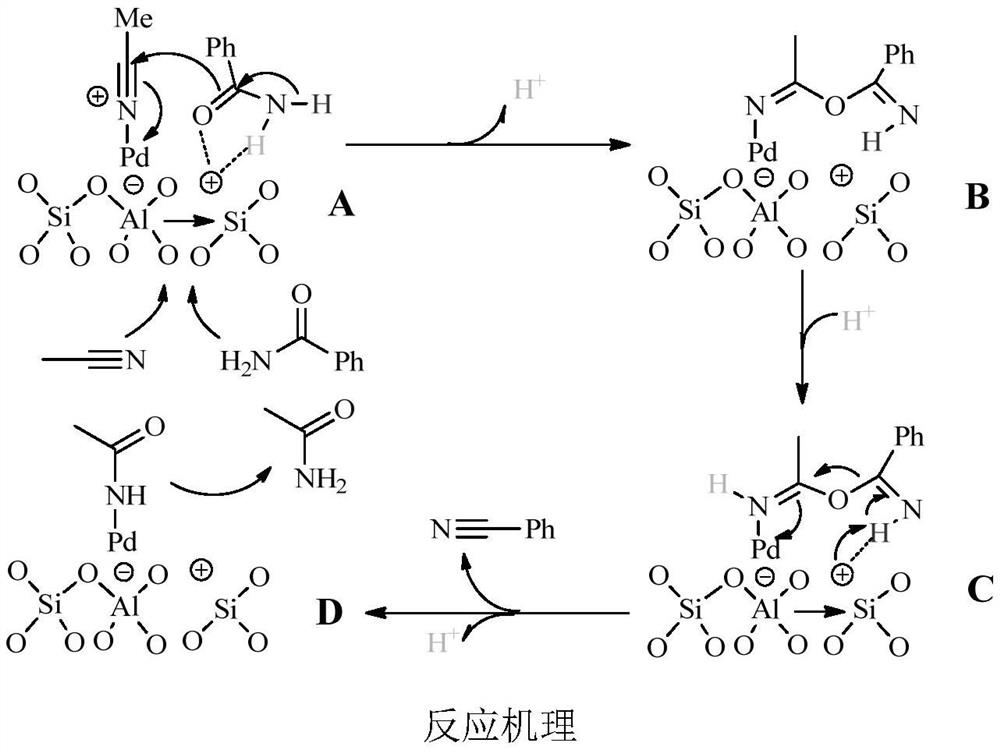
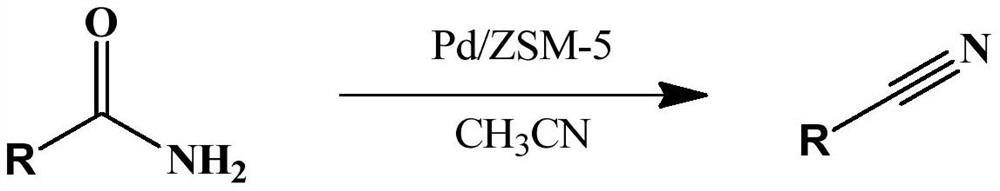A kind of preparation method for synthesizing aromatic nitrile catalyst and the synthetic method of aromatic nitrile
A synthesis method and catalyst technology, which are applied in molecular sieve catalysts, chemical instruments and methods, physical/chemical process catalysts, etc., can solve the problems of increasing synthesis cost, large environmental impact, and difficulty in obtaining metal complex catalysts, and achieve easy and efficient products. Excellent catalytic performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0029] The preparation method of Pd / ZSM-5 is:
[0030] Weigh 2.0g of ZSM-5 carrier into a 150mL beaker, add 80mL of distilled water, weigh 0.1026g of Pd(NO) 3 ) 2 ·2H 2 O was added to the beaker and stirred at room temperature for 72h. After filtration and washing, the obtained catalyst sample was dried in an oven at 50° C. for 72 h to obtain a Pd / ZSM-5 catalyst.
specific Embodiment 1
[0031] Benzamide (2 mmol, 0.24 g), Pd / ZSM-5 (0.1 g) and 20 mL of acetonitrile were sequentially added to a 50 mL reaction kettle, the reaction temperature was controlled at 100 °C, and the reaction was vigorously stirred for 4 h. After the reaction was completed, it was cooled to room temperature, the reaction system was centrifuged, and the reaction solution was subjected to chromatographic analysis. All the reactants were converted, and the selectivity of benzonitrile was 100%; After several experiments, yields of 92-97% were obtained).
[0032] The equation involved in the reaction is as follows:
[0033]
specific Embodiment 2
[0034] Benzamide (2 mmol, 0.24 g), Pd / ZSM-5 (0.1 g) and 20 mL of acetonitrile were sequentially added to a 50 mL reaction kettle, the reaction temperature was controlled at 80 °C, and the reaction was vigorously stirred for 6 h. After the reaction was completed, it was cooled to room temperature, the reaction system was centrifuged, and the reaction solution was analyzed by chromatography. All the reactants were converted, and the selectivity of benzonitrile was 100%. Benzonitrile can be obtained by distillation under reduced pressure, and benzonitrile ~ 0.19 g (yield 92-97%) can be obtained by distillation under reduced pressure.
[0035] The equation involved in the reaction is as follows:
[0036]
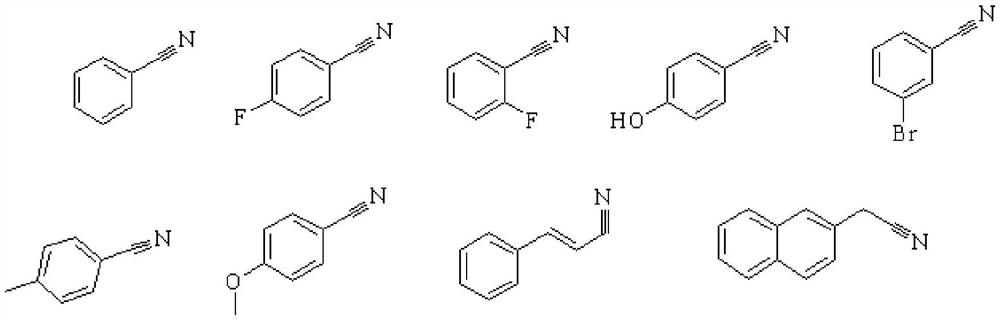
[0037] The above-mentioned catalysts, under the same reaction conditions, partially expand R, all obtain better catalytic effects: higher conversion rate and product selectivity, and the target product and catalytic performance are:
[0038]
PUM
| Property | Measurement | Unit |
|---|---|---|
| specific surface area | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com