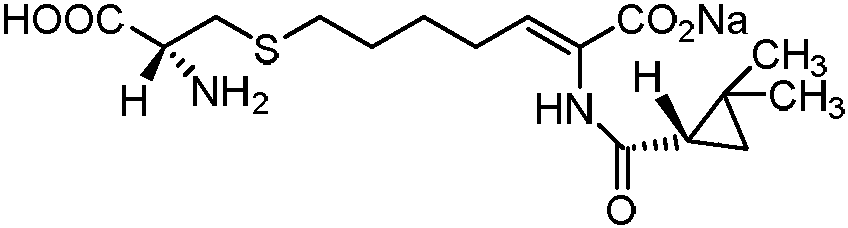
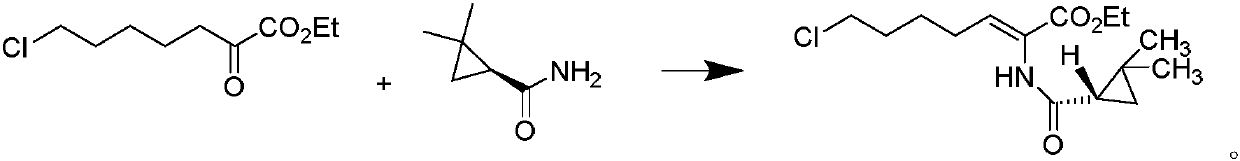A kind of preparation method of cilastatin sodium intermediate
A technology of cilastatin sodium and intermediates, which is applied in the field of preparation of cilastatin sodium intermediates, can solve the problems of long reflux reaction time, long hydrolysis process time, unfavorable industrial recovery, etc., and achieve good product stability, The effect of less impurities and saving production cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
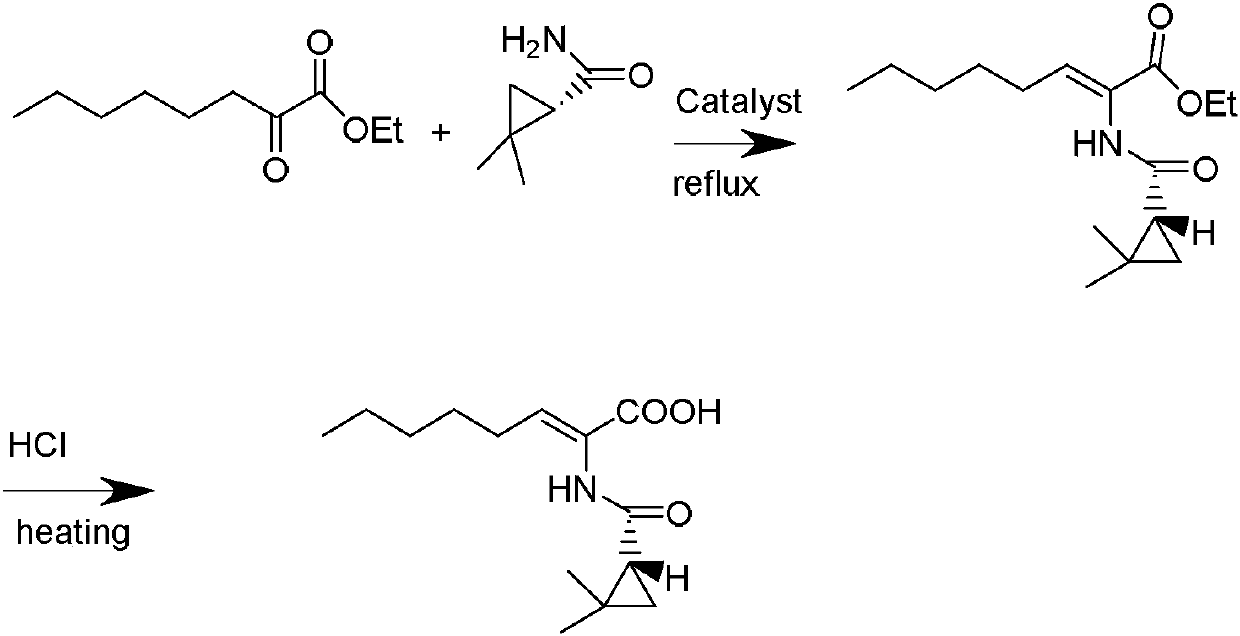
[0036] Example 1: Preparation of (Z)-7-chloro-2((2s)-2,2-dimethylcyclopropanecarboxamido)-2-heptenoic acid
[0037] 25.0 g (121 mmol) of ethyl 7-chloro-2-oxoheptenoate, 13.68 g (121 mmol) of (S)-2,2-dimethylcyclopropanecarboxamide, and 125 ml of toluene were added to a 500 ml flask, Stir, then add 0.5 g of concentrated sulfuric acid, heat up to reflux, continuously remove the generated water, keep warm and reflux for 5 hours. Cool down to 70°C, slowly add 50ml of concentrated hydrochloric acid, slowly pass in HCl gas until the reaction is complete, keep stirring for 2h. Stop the flow of hydrogen chloride gas, lower the temperature to -10°C, keep stirring at this temperature for 7 hours, and TLC detects that the impurity spots completely disappear. The organic phase was washed successively with saturated brine (2×100ml) and purified water (2×100ml), extracted, dried over anhydrous sodium sulfate, filtered, and the toluene was evaporated under reduced pressure at 50°C to obtain...
Embodiment 2
[0038] Example 2: Preparation of (Z)-7-bromo-2((2s)-2,2-dimethylcyclopropanecarboxamido)-2-heptenoic acid
[0039] 30.39g (121mmol) of ethyl 7-bromo-2-oxoheptenoate, 13.68g (121mmol) of (S)-2,2-dimethylcyclopropanecarboxamide, and 125ml of toluene were added to a 500ml flask, After stirring, 0.31 g of p-toluenesulfonic acid was added, the temperature was raised to reflux, and the generated water was continuously separated, and the temperature was kept at reflux for 6 hours. Cool down to 65°C, slowly add 75ml of concentrated hydrochloric acid, slowly inject HCl gas until the reaction is complete, and keep stirring for 1.5h. Stop the flow of hydrogen chloride gas, lower the temperature to -15°C, keep stirring at this temperature for 4 hours, and TLC detects that the impurity spots completely disappear. The organic phase was washed successively with saturated brine (2×100ml) and purified water (2×100ml), extracted, dried over anhydrous sodium sulfate, filtered, and the toluene w...
Embodiment 3
[0040] Example 3: Preparation of (Z)-7-chloro-2((2s)-2,2-dimethylcyclopropanecarboxamido)-2-heptenoic acid
[0041] 35.72g (121mmol) of 7-chloro-2-oxoheptenoic acid butyl ester, (S)-2, 13.68g (121mmol) of 2-dimethylcyclopropanecarboxamide, 125ml of toluene were added in a 500ml flask, After stirring, 1.79g of benzenesulfonic acid was added, the temperature was raised to reflux, and the generated water was continuously separated, and the temperature was kept at reflux for 4 hours. Cool down to 75°C, slowly add 179ml of concentrated hydrochloric acid, slowly inject HCl gas until the reaction is complete, and keep stirring for 3.5h. Stop the flow of hydrogen chloride gas, lower the temperature to -6°C, keep stirring at this temperature for 12 hours, and TLC detects that the impurity spots completely disappear. The organic phase was washed successively with saturated brine (2×100ml) and purified water (2×100ml), extracted, dried over anhydrous sodium sulfate, filtered, and the to...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com