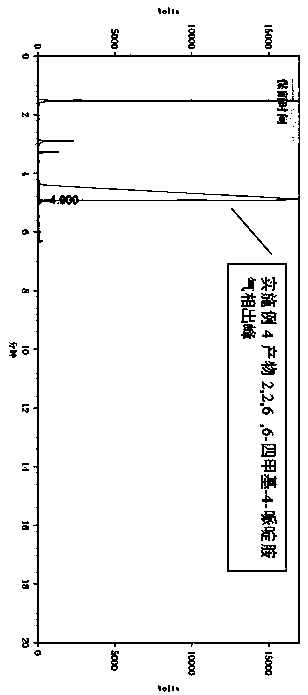
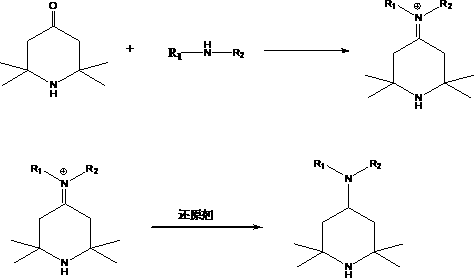
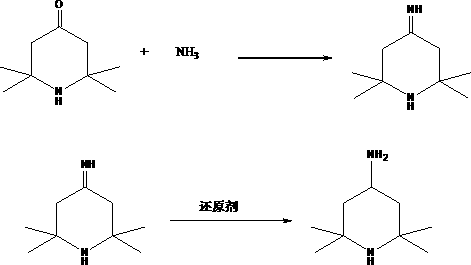
2,2,6,6-tetramethyl-4-piperidinamine preparation method
A technology of piperidine amine and compounds, which is applied in the field of chemical synthesis, can solve the problems of tightening safety and environmental protection policies, potential safety hazards, and high cost, and achieve the effects of eliminating potential safety hazards, reducing danger, and eliminating flammability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] In a 500ml four-necked flask equipped with a thermometer, add 155g of 2,2,6,6-tetramethylpiperidone, 200g of water, install a condenser, control the temperature at 20-30°C, and control the pressure at 0-1Mpa. Pass 40g of liquid ammonia through the way of passing amine under the liquid, stir while passing through, and after stirring for 0.5 hours, raise the temperature to 50°C, use a dropping funnel to drop 160g of 50% mass fraction of formic acid aqueous solution, and keep it warm for 4 hours after dropping. Then the excess formic acid and solvent were evaporated by heating up, and then the product tetramethylpiperidinamine was obtained through rectification under reduced pressure, and the product yield was 92%.
Embodiment 2
[0035]Put 155g of 2,2,6,6-tetramethylpiperidone, 100g of water, and 100g of methanol into the autoclave at one time, then tighten the autoclave, replace with nitrogen for 3 times, and stir at 20-30°C, Pour in 50g of liquid ammonia, stir for half an hour, then pour in 184g of 50% formic acid aqueous solution, then raise the temperature to 90°C, control the pressure at 0.2-0.5Mpa, react for 3 hours, then cool down. The reaction solution was transferred to a flask, and then rectified after desolvation to obtain the product tetramethylpiperidinamine with a product yield of 94%.
Embodiment 3
[0037] Put 155g of 2,2,6,6-tetramethylpiperidone, 100g of methanol, and 130g of n-butylamine into the autoclave at one time, then tighten the autoclave, replace with nitrogen for 3 times, and stir at 40-50°C for 1 hour , and then pass 54g of formic acid aqueous solution with 85% mass fraction, raise the temperature to 120°C, the pressure does not exceed 2Mpa, stir and keep warm for 4 hours, then cool down to remove the solvent from the reaction solution, and finally vacuum distill the product N-butyl-2,2 , 6,6-tetramethyl-4-piperidinamine, the product yield is 92%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com