A kind of production technology of carfilzomib freeze-dried preparation for injection
A technology of carfilzomib and freeze-dried preparations, which is applied in the field of medicine, can solve the problems of increased risk of clinical medication, high levels of related substances, and long time for liquid preparation, and achieve flexible production and operation time, low endotoxin, and extended storage The effect of the time limit
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
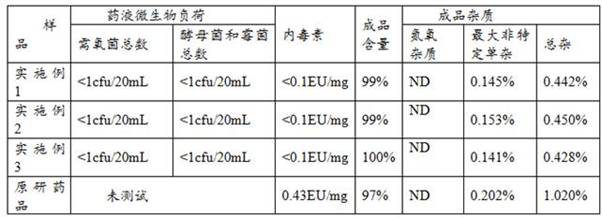
Embodiment 1
[0027] A production process of carfilzomib freeze-dried preparation for injection, comprising the steps of:
[0028] A. At room temperature, add water for injection into the liquid preparation tank, then add 150 parts by weight of sulfobutyl-β-cyclodextrin, and stir until dissolved;
[0029] B. Add 2.5 parts by weight of anhydrous citric acid, stir until dissolved, and fill the solution with nitrogen;
[0030] C. Add 3 parts by weight of carfilzomib bulk drug under stirring conditions, stir and sonicate until dissolved, and reduce the temperature of the drug solution to 2°C;
[0031] D, slowly adding NaOH solution to adjust the pH to 3.0, and set the volume to 1000 parts by weight with water for injection;
[0032] E. After the liquid preparation is completed, immediately pre-filter the liquid medicine into a buffer tank, and fill the headspace with nitrogen to maintain pressure;
[0033] F. Sterilization and filtration, aseptic filling, freeze-drying, nitrogen repressurizat...
Embodiment 2
[0036] A production process of carfilzomib freeze-dried preparation for injection, comprising the steps of:
[0037] A. At room temperature, add water for injection into the liquid preparation tank, then add 200 parts by weight of sulfobutyl-β-cyclodextrin, and stir until dissolved;
[0038] B. Add 4 parts by weight of anhydrous citric acid, stir until dissolved, fill the solution with nitrogen, and fill the solution with nitrogen until the dissolved oxygen content is less than 1ppm. ;
[0039] C. Add 4 parts by weight of the carfilzomib bulk drug under stirring conditions, stir and sonicate until dissolved, and reduce the temperature of the drug solution to 8°C;
[0040] D, slowly adding NaOH solution to adjust the pH to 4.0, and set the volume to 1000 parts by weight with water for injection;
[0041] E. After the liquid preparation is completed, immediately pre-filter the liquid medicine into a buffer tank, and fill the headspace with nitrogen to maintain pressure;
[00...
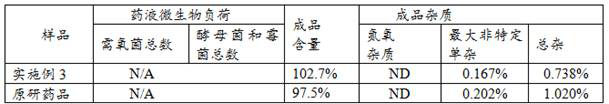
Embodiment 3
[0047] A production process of carfilzomib freeze-dried preparation for injection, comprising the steps of:
[0048] A. At room temperature, add water for injection into the liquid preparation tank, then add 180 parts by weight of sulfobutyl-β-cyclodextrin, and stir until dissolved;
[0049] B. Add 3 parts by weight of anhydrous citric acid, stir until dissolved, fill the solution with nitrogen, and fill the solution with nitrogen until the dissolved oxygen content is less than 1ppm. ;
[0050] C. Add 3.5 parts by weight of the carfilzomib bulk drug under stirring conditions, stir and sonicate until dissolved, and reduce the temperature of the drug solution to 3°C;
[0051] D, slowly adding NaOH solution to adjust the pH to 3.5, and set the volume to 1000 parts by weight with water for injection;
[0052] E. After the liquid preparation is completed, immediately pre-filter the liquid medicine into a buffer tank, and fill the headspace with nitrogen to maintain pressure;
[00...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com