Application of vanillin as redox medium in increasing diethylstilbestrol degradation rate of laccase
A technology of diethylstilbestrol and vanillin, which is applied in the field of environmental pollution treatment, can solve problems such as insignificant effect, slow down photodegradation, and poor effect, and achieve the effects of overcoming steric hindrance and kinetic limitations, simple operation, and low cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
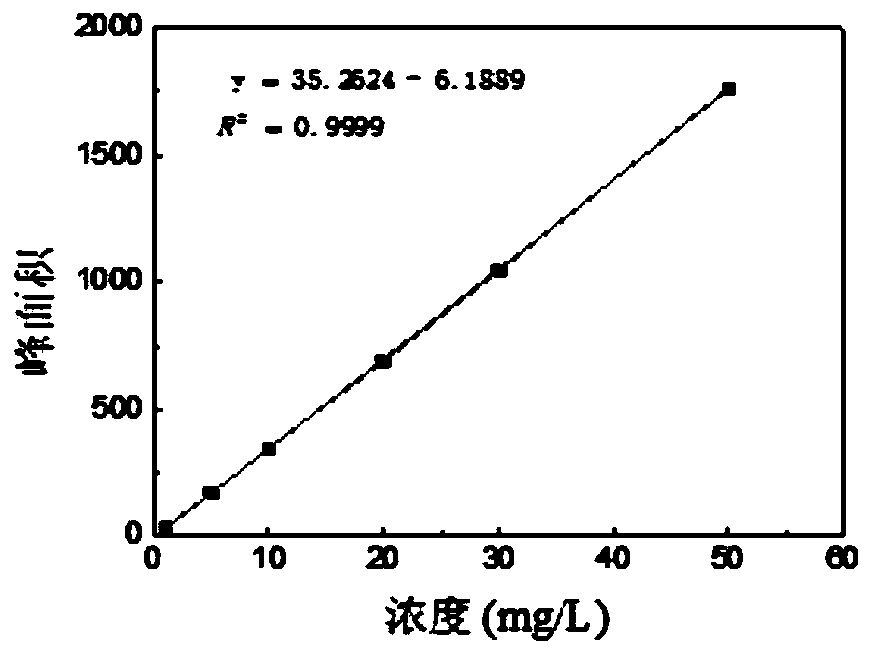
[0031] Draw Diethylstilbestrol Standard Curve
[0032] Diethylstilbestrol mother liquor with a concentration of 2.5g / L was diluted to obtain standard solutions of diethylstilbestrol with a concentration of 1mg / L, 5mg / L, 10mg / L, 20mg / L, 30mg / L, and 50mg / L respectively, and the HPLC detection method was used to draw Diethylstilbestrol standard curve, such as figure 1 shown.
[0033] HPLC detection conditions are chromatographic column: ZORBAX SB-C18 (150mm×4.6mm×5μm); detection conditions are: injection volume 10μL, elution with equal volume of acetonitrile and water as flow equal gradient for 10min, pump flow rate is 1.0mL / min, column temperature: 30°C, the ultraviolet detector detects the peak at 240nm wavelength, and records the peak area value.
Embodiment 2
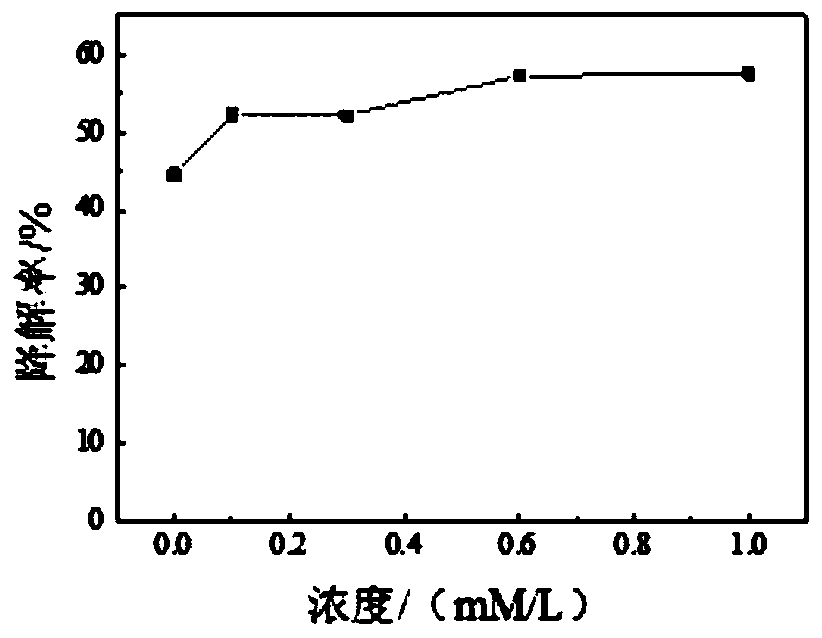
[0035] (1) Mix 0.5mL 1mg / mL laccase mother solution, 0mL 0.01mol / L vanillin solution and 0.4mL 2.5g / L DES mother solution, supplement to 10mL with acetic acid-sodium acetate buffer solution, and place at 55°C , 120rpm shaking incubator reaction.
[0036] (2) After reacting for half an hour, use ethyl acetate to extract the solution twice, extract the organic phase and then evaporate to dryness, use methanol to constant volume, use vacuum filter membrane to sample, and measure the concentration of the reacted sample by HPLC. Measure and calculate the degradation rate.
[0037] Degradation rate calculation formula: degradation rate = (C 0 -C) / C 0 ×100%, where C 0 is the initial concentration and C is the final concentration.
[0038] After calculation, the degradation rate of this embodiment is 44.56%.
Embodiment 3
[0040](1) Mix 0.5mL 1mg / mL laccase mother solution, 0.1mL 0.01mol / L vanillin solution and 0.4ml 2.5g / L DES mother solution, supplement to 10mL with acetic acid-sodium acetate buffer solution, and place at 55°C , 120rpm shaking incubator reaction.
[0041] (2) After reacting for half an hour, use ethyl acetate to extract the solution twice, extract the organic phase and then evaporate to dryness, use methanol to constant volume, use vacuum filter membrane to sample, and measure the concentration of the reacted sample by HPLC. Measure and calculate the degradation rate.
[0042] Degradation rate calculation formula: degradation rate = (C 0 -C) / C 0 ×100%, where C 0 is the initial concentration and C is the final concentration.
[0043] After calculation, the degradation rate of this embodiment is 52.25%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com