Synthesis method of natural anisaldehyde
A technology of natural anethole and synthesis method, which is applied in chemical instruments and methods, preparation of carbon-based compounds, preparation of organic compounds, etc., can solve the problems of high investment cost, limited promotion, etc., and achieves reduction of solid waste, cost reduction, less corrosive effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0022] According to the present invention object is to provide a kind of synthetic method of anisaldehyde, this method comprises the following steps:
[0023] Step 1. Add natural anethole, catalyst and solvent into the reaction vessel, mix and heat up.
[0024] In step 1, the anethole and the solvent are firstly added into the reaction vessel, stirred and mixed evenly, and then the catalyst is added, and evenly dispersed in the reaction solution under the stirring condition.
[0025] In a preferred mode of the present invention, the reaction vessel is connected to a reflux device for cooling and refluxing the volatilized solvent during the heating reaction.
[0026] The purity of the natural anethole should not be lower than 90%. If the content of the raw material anethole is too low, impurities will be introduced from the source, which will make it more difficult to store the product anisaldehyde, so that the purity of the product cannot meet the requirements for use.
[002...
Embodiment Embodiment 1
[0061] Put 148g of natural anethole (the purity of anethole is 90%) into the reaction vessel, then add 200g of methanol and mix well, then add 1.5g of cobalt acetate, 0.5g of copper acetate and 2.0g of disodium hydrogen phosphate The mixture was stirred and dispersed, and the reaction system was heated to 50°C. Then, after the oxygen was treated by the micro-nano bubble generator, it was passed into the flask by the gas distributor to carry out the oxidation reaction. The oxygen flow rate was controlled at 200mL / min, and the reflux reaction was started. . Until the mass fraction of anethole is less than 1%, the heating is stopped, and the reaction is terminated, and the reaction time is 6 hours. The conversion rate of anethole was sampled and tested to be 99%, and the selectivity of anisaldehyde was 90.8%.
[0062] Cobalt acetate, copper acetate and disodium hydrogen phosphate are washed out with water, and the recovered catalyst is applied to the next batch of reactions. Th...
Embodiment 2
[0067] Carry out experiment by embodiment 1, difference is only: catalyst is 1.5g cobalt acetate, 0.5g copper acetate and 2.0g dipotassium hydrogen phosphate. The reaction time was 6 hours, and the product was distilled under reduced pressure to obtain 118.2 g (purity: 95.3%). The conversion rate and selectivity of anisehole were 99.5% and 92.4%, respectively, and the yield of anisaldehyde was 92%.
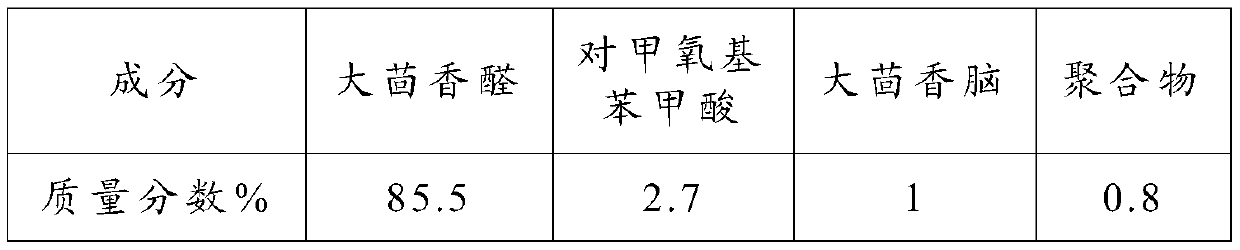
[0068] Qualitative and quantitative analysis was performed on the components of the distillate after solvent separation by chromatography-mass spectrometry.
[0069] The test results are shown in the table below:
[0070] Product composition and content in the embodiment 2 of table 2
[0071]
[0072] As can be seen from the experimental results in Example 1 and Example 2, when disodium hydrogen phosphate and dipotassium hydrogen phosphate were co-catalysts, the conversion rate and selectivity of the reaction system did not change much, indicating that the main role played in...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com