Nano fluorescent probe, and preparation method and application thereof in detection of HNO in Golgi apparatus
A nano-fluorescent probe, fluorescein technology, used in fluorescence/phosphorescence, chemical instruments and methods, luminescent materials, etc., can solve the problems of short existence time, limitation, difficult capture, etc., and achieve low phototoxicity, cell and living body damage. The effect of small, simple synthetic route
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0045] Synthesis of fluorescent probes
[0046] Dissolve the raw material 2-diphenylphosphobenzoic acid (5mmol), dicyclohexylcarbodiimide (5mmol), and 4-dimethylaminopyridine (0.25mmol) in 15mL of dichloromethane, activate the carboxyl group at 0°C for 30min, Fluorescein (1 mmol) was then added and stirred at room temperature for 12 h. After the reaction was complete, the solvent was removed by rotary evaporation. Then using dichloromethane:methanol=20:1 as eluent, purified by column chromatography to obtain yellow solid C-HNO (57%).
[0047]Dissolve 2 mg of C-HNO in 300 μL of dimethyl sulfoxide, quickly add it into 100 mg of BSA previously dissolved in 10 mL of deionized water with a pipette, and stir at room temperature for 1 hour. After the reaction was stopped, dialyze overnight with a dialysis bag with a molecular weight of 3000 to obtain an aqueous solution of nano fluorescent probe BSA-HNO with a concentration of 100 μg / mL.
[0048] Effect experiment:
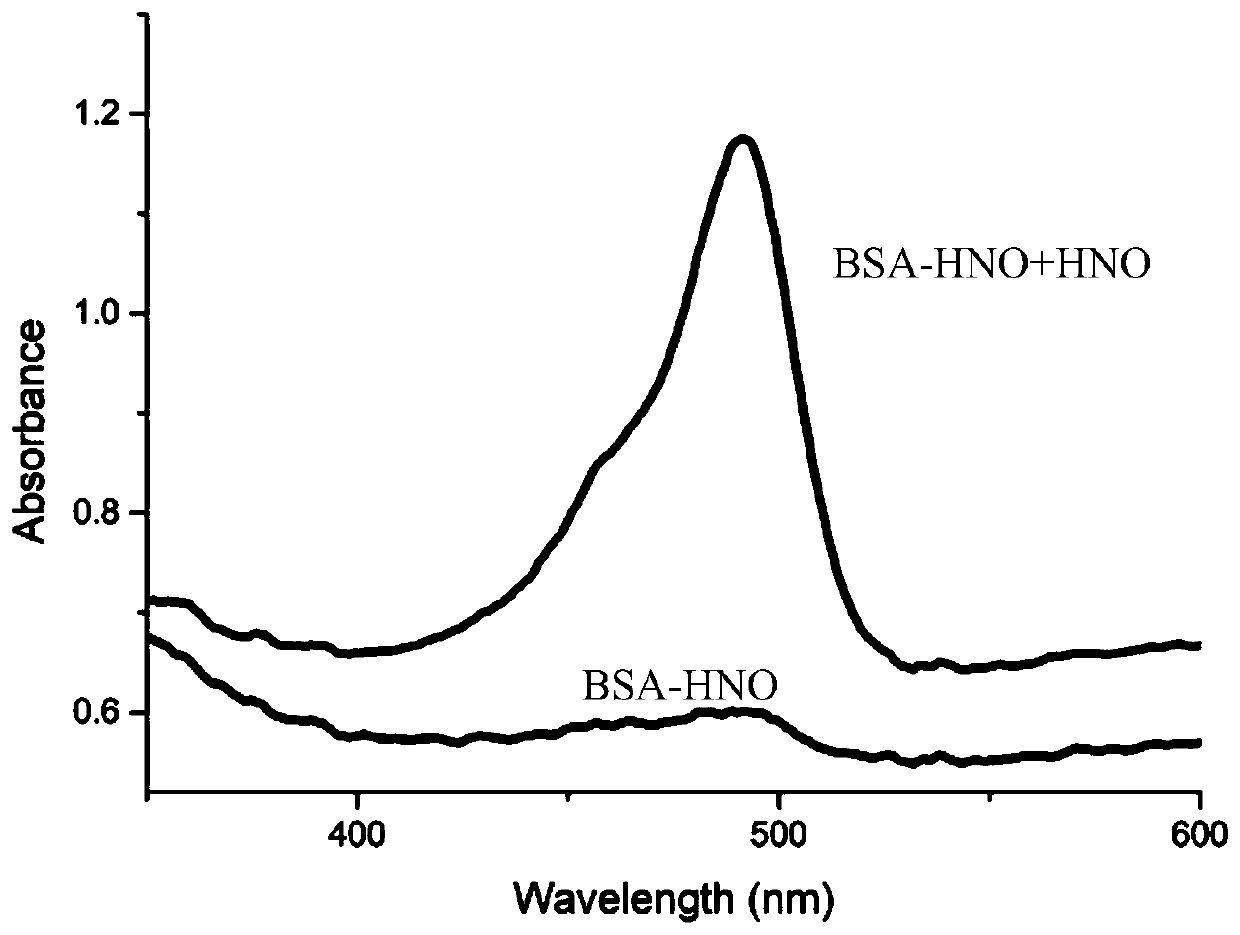
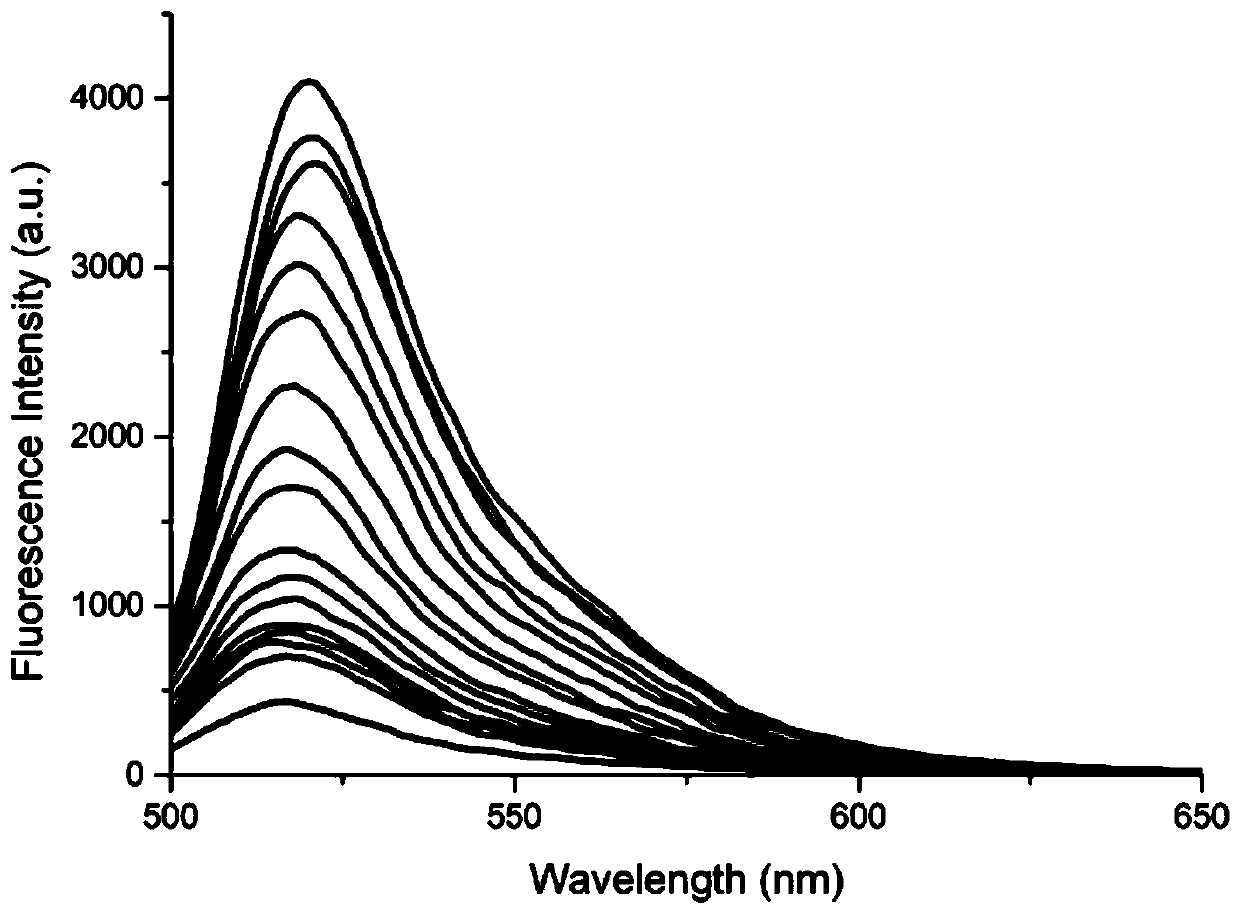
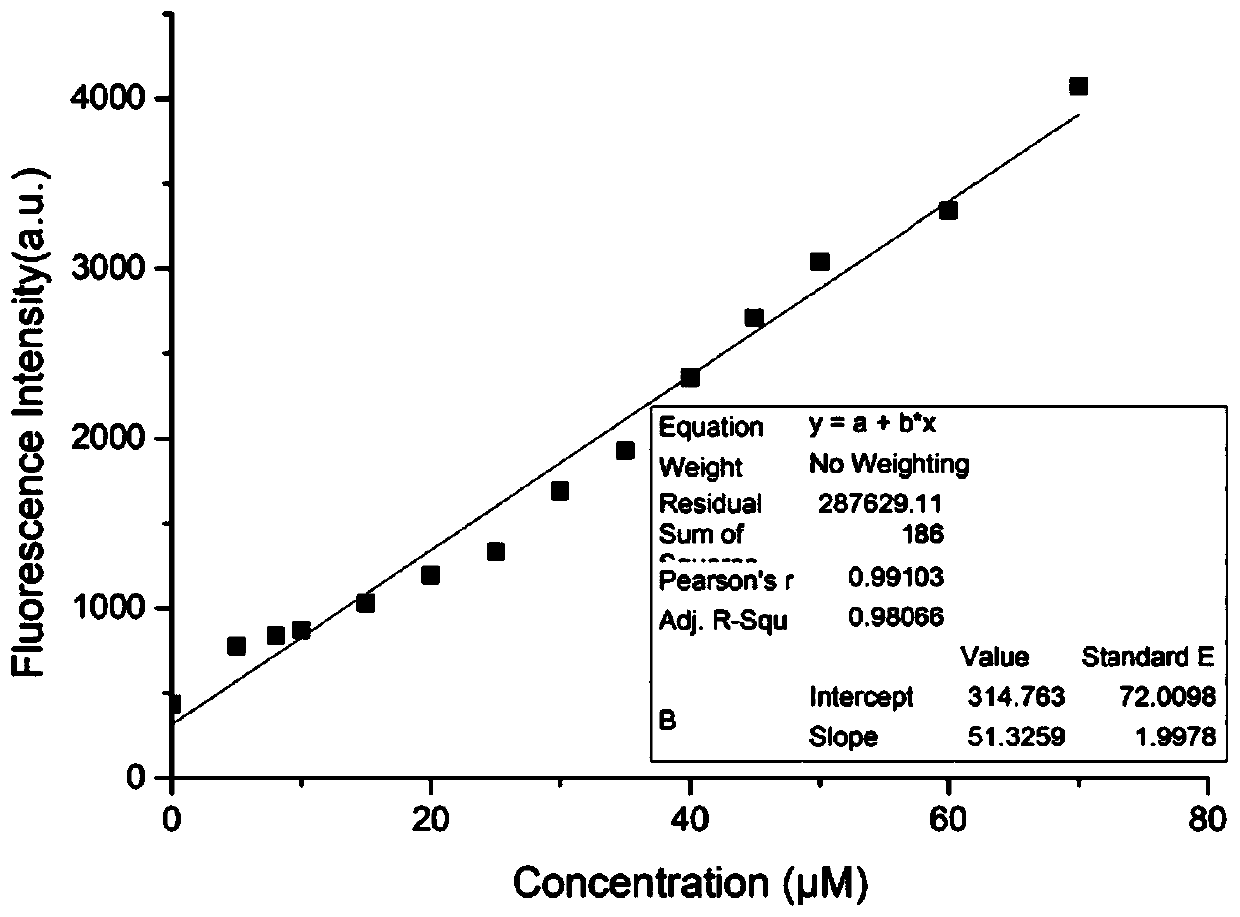
[0049] Gener...
Embodiment 2
[0056] Synthesis of fluorescent probes
[0057] Dissolve the raw materials 2-diphenylphosphobenzoic acid (1mmol), dicyclohexylcarbodiimide (1mmol), and 4-dimethylaminopyridine (0.05mmol) in 15mL of dichloromethane, activate the carboxyl group at 0°C for 30min, Fluorescein (1 mmol) was then added and stirred at room temperature for 12 h. After the reaction was complete, the solvent was removed by rotary evaporation. Then using dichloromethane:methanol=20:1 as eluent, purified by column chromatography to obtain yellow solid C-HNO (15%).
[0058] Dissolve 2 mg of C-HNO in 300 μL of dimethyl sulfoxide, quickly add it into 100 mg of BSA previously dissolved in 10 mL of deionized water with a pipette, and stir at room temperature for 1 hour. After the reaction was stopped, dialyze overnight with a dialysis bag with a molecular weight of 3000 to obtain an aqueous solution of nano fluorescent probe BSA-HNO with a concentration of 100 μg / mL.
Embodiment 3
[0060] Synthesis of fluorescent probes
[0061] Dissolve the raw material 2-diphenylphosphobenzoic acid (3mmol), dicyclohexylcarbodiimide (3mmol), and 4-dimethylaminopyridine (0.15mmol) in 15mL of dichloromethane, activate the carboxyl group at 0°C for 30min, Fluorescein (1 mmol) was then added and stirred at room temperature for 12 h. After the reaction was complete, the solvent was removed by rotary evaporation. Then using dichloromethane:methanol=20:1 as eluent, purified by column chromatography to obtain yellow solid C-HNO (24%).
[0062] Dissolve 2 mg of C-HNO in 300 μL of dimethyl sulfoxide, quickly add it into 100 mg of BSA previously dissolved in 10 mL of deionized water with a pipette, and stir at room temperature for 1 hour. After the reaction was stopped, dialyze overnight with a dialysis bag with a molecular weight of 3000 to obtain an aqueous solution of nano fluorescent probe BSA-HNO with a concentration of 100 μg / mL.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com