A kind of esterified cholic acid/Fe3O4 magnetic nanocomposite and its preparation method and application
A technology of esterified cholic acid and magnetic nanotechnology, which is applied in the direction of pharmaceutical formulations, drug combinations, organic active ingredients, etc., can solve problems such as adverse reactions, achieve the effects of reducing toxic and side effects, strong biocompatibility, and achieving targeting
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
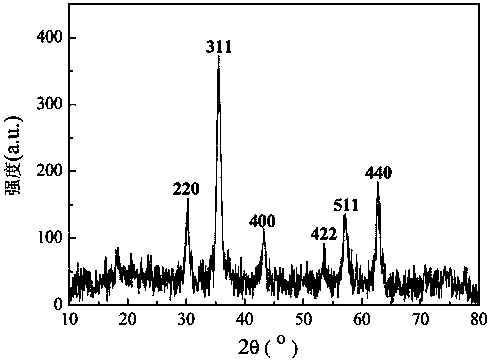
[0026] Example 1: TC-APTES-Fe 3 o 4 Preparation of MNPs
[0027] (1) Fe 3 o 4 Preparation of MNPs: Weigh 1 g of FeCl 2 4H 2 O and 2.7 g FeCl 3 ·6H 2 O was placed in the reactor, and 200 mL of secondary water was added. Under the protection of nitrogen, heat to 80°C, stir mechanically until dissolved; meanwhile, add concentrated ammonia water (25%) dropwise to adjust the pH of the reaction solution to 10, and react at 800r / min for 2 hours. After the reaction was completed, the reaction solution was cooled to room temperature and separated by magnetic hysteresis. The dark brown precipitate was washed with secondary water for 3 times, and then repeatedly washed with ethanol (at least 3 times) until neutral. Vacuum drying at 60°C for 12 hours yielded Fe 3 o 4 MNPs;
[0028] (2) APTES-Fe 3 o 4 Preparation of MNPs: 300mg of Fe 3 o 4 MNPs were ultrasonically dispersed in 600mL ethanol solution, and 120uL APTES was added under nitrogen protection. Stir mechanically at ...
Embodiment 2
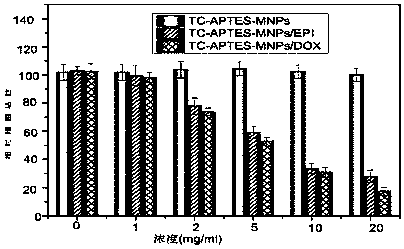
[0038] Example 2: pH versus TC-APTES-Fe 3 o 4 Effect of MNPs drug loading performance
[0039] Weigh 7 portions of 2mg TC-APTES-Fe 3 o 4 For MNPs, add 4mL of phosphate buffer with different pH (3-9) and 4mL concentration of 1×10 -5 mol / L drug DOX. Sonicate for 10 minutes, and shake the solution at room temperature for 24 hours. After the reaction was completed, hysteresis separation was performed, and the fluorescence intensity of the supernatant was measured at 491 nm. Data processing to explore the effect of pH on TC-APTES-Fe 3 o 4 -Influence of the drug-loading performance of MNPs (TC-APTES-Fe 3 o 4 The experiment of MNPs on drug EPI drug loading performance is the same as DOX). Such as Figure 7 As shown, when pH≤8, TC-APTES-Fe 3 o 4 The loading of DOX and EPI by MNPs increased with the increase of pH, and the loading increased significantly at pH=7. When the pH was 8-9, the loading capacity decreased slightly again. In summary, neutral conditions are more f...
Embodiment 3
[0040] Example 3: TC-APTES-Fe 3 o 4 Preparation of inclusion complexes between MNPs and doxorubicin hydrochloride (DOX) or epirubicin hydrochloride (EPI)
[0041] Weigh 20mg TC-APTES-Fe 3 o 4 MNPs, add 4mL pH = 7.4 phosphate buffer solution, ultrasonically mix, then add 4mL concentration of 10 -5 mol / L DOX, sonicate at room temperature for 10 minutes, shake for 24 hours; magnetic hysteresis separation (measure the fluorescence intensity after diluting the supernatant by a certain number of times, and determine the drug content in the clathrate), and vacuum-dry the precipitate at 60°C for 12 hours , get TC-APTES-Fe 3 o 4 Inclusion complexes of MNPs and drug DOX (preparation conditions of EPI inclusion complexes are the same as those of DOX inclusion complexes). The fluorescence intensity of the supernatant was measured at 491nm, and the calculated actual drug loads of DOX and EPI clathrates were 198.44mg / g and 198.6mg / g, respectively.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com