Recombinant adeno-associated virus vector carrying human sperm protein 17 antigen gene and application value thereof
A virus carrier and antigen gene technology, applied in the direction of virus/bacteriophage, application, carrier, etc., can solve the problems of insufficient ability, low efficiency, poor effect, etc., and achieve the effect of broad application prospects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] Example 1, Construction and identification of SP17 recombinant adeno-associated virus vector
[0038] 1. Materials and their sources:
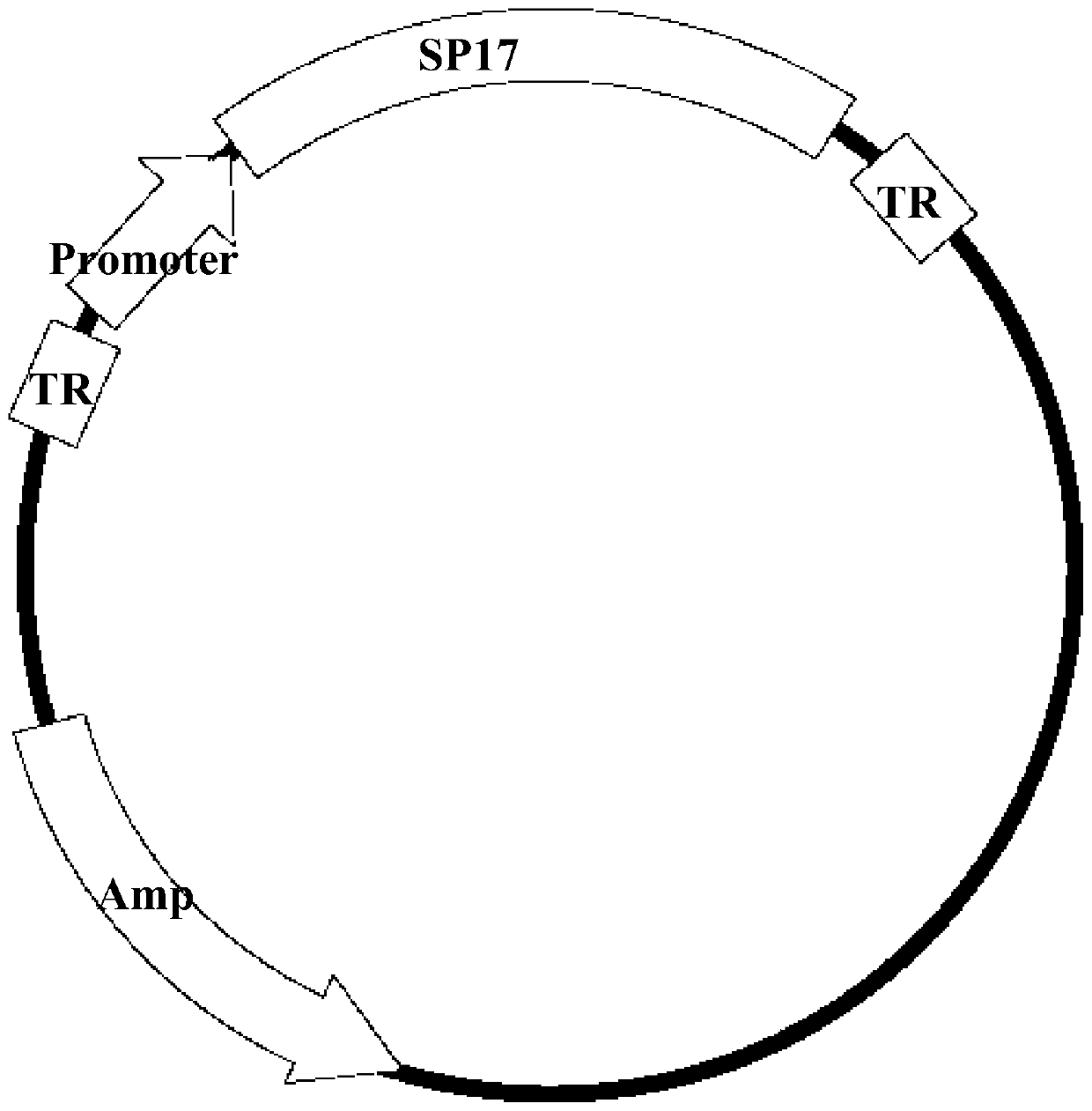
[0039] 1. Four kinds of AAV type 2 (AAV-2) pBR322 plasmids (pBR-AAV2) with different promoters: the plasmids were successfully constructed by the inventors (see Chinese patent ZL201110125683.X, 0056-0059 section pBR-AAV2 plasmid reconstruction , PCR amplification of the promoter, inserting the amplified promoter into the rebuilt pBR-AAV2 plasmid, etc.). The four promoters are AAV p5 promoter (AAV p5), macrophage virus (CMV) promoter (CMVp), SV40 early promoter (SV40p) and human β-actin (β-actin) promoter ( β-actinp). The feature of this plasmid is the complete repeat terminal segment (TR) sequence at both ends, and a fragment CTGCGCTGG consisting of 9 nucleotides is inserted at the 75th nucleotide sequence of both ends TR, the purpose is to improve the recombinant AAV virus ( rAAV) and improve the replication efficiency of the virus,...
Embodiment 2
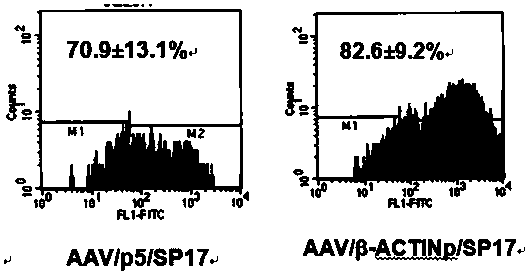
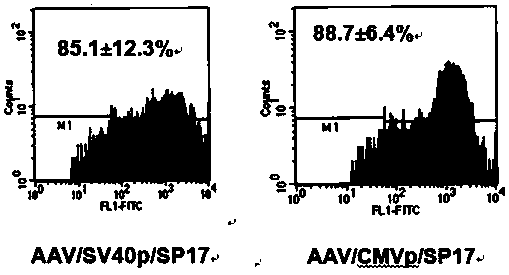
[0052]Embodiment 2, the preparation of the viral vector of recombinant adeno-associated virus (rAAV) and titer determination (such as figure 2 shown)
[0053] Materials and their sources:
[0054] A. The recombinant adeno-associated virus plasmid vector carrying the SP17 antigen gene constructed in Example 1.
[0055] B. The auxiliary plasmid pHelper containing the Rep gene and Lip / Cap gene of AAV: constructed by the inventors of this patent application (Liu, Y., Chiriva-Internati, M., Grizzi, F.Salati, E., Roman, J.J., Lim S., and Hermonat, P.L. Rapid induction of cytotoxic T cell response against cervical cancer cells by human papillomavirus type 16 E6 antigen gene delivery into human dendritic cells by an adeno-associated virus vector. Cancer Gene Therapy 8:948-957.).
[0056] C. AAVp cell line containing adenovirus genes (E1, E2A, E4, VAI and VAII genes) integrated in the cell chromosome and expressed: established by the Gene Therapy Center of the University of Arkansas...
Embodiment 3
[0072] Example 3. Experiment of lysing SP17 antigen-positive malignant tumor cells by introducing recombinant adeno-associated virus carrying SP17 antigen gene into monocyte-dendritic cell line
[0073] Materials and their sources:
[0074] A. Recombinant adeno-associated virus carrying the SP17 antigen gene.
[0075] B. AIM-V cell culture medium: purchased from Life Technologies, USA.
[0076] C. Cytokines: colony cell stimulating factor (GM-CSF), interleukin 2, 4 (IL-2, IL-4) and tumor necrosis factor (TNF-α) were purchased from American R&D Corporation.
[0077] D. Primary tumor cells positive for SP17 antigen: tumor cells isolated from tumor tissues of multiple myeloma and renal carcinoma respectively, and immunohistochemistry has confirmed that they are positive for SP17 antigen.
[0078] E. SP17 antigen-negative primary cells: tumor cells isolated from tumor tissues of skin epithelial cell carcinoma, lung adenocarcinoma, kidney cancer and multiple myeloma, immunohistoc...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com