High-activity Ni/ACP catalyst and preparation method and application thereof
A catalyst, high activity technology, used in catalyst activation/preparation, physical/chemical process catalysts, catalysts, etc., can solve problems such as low catalytic activity, unfavorable metal dispersion, and small catalyst specific surface area.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
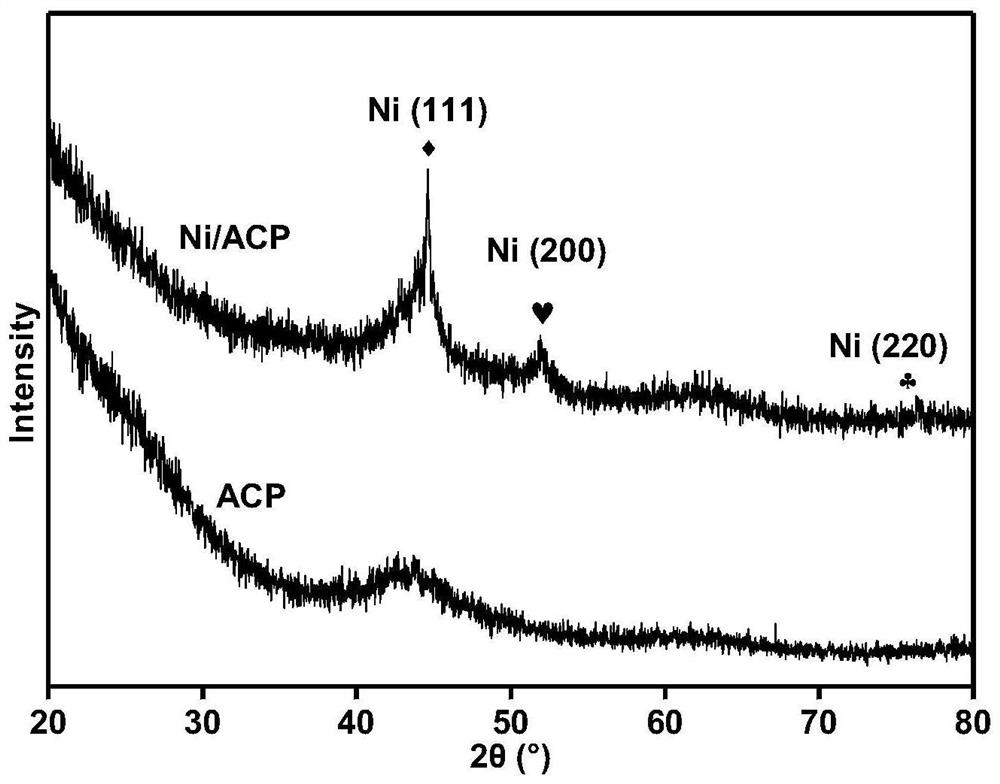
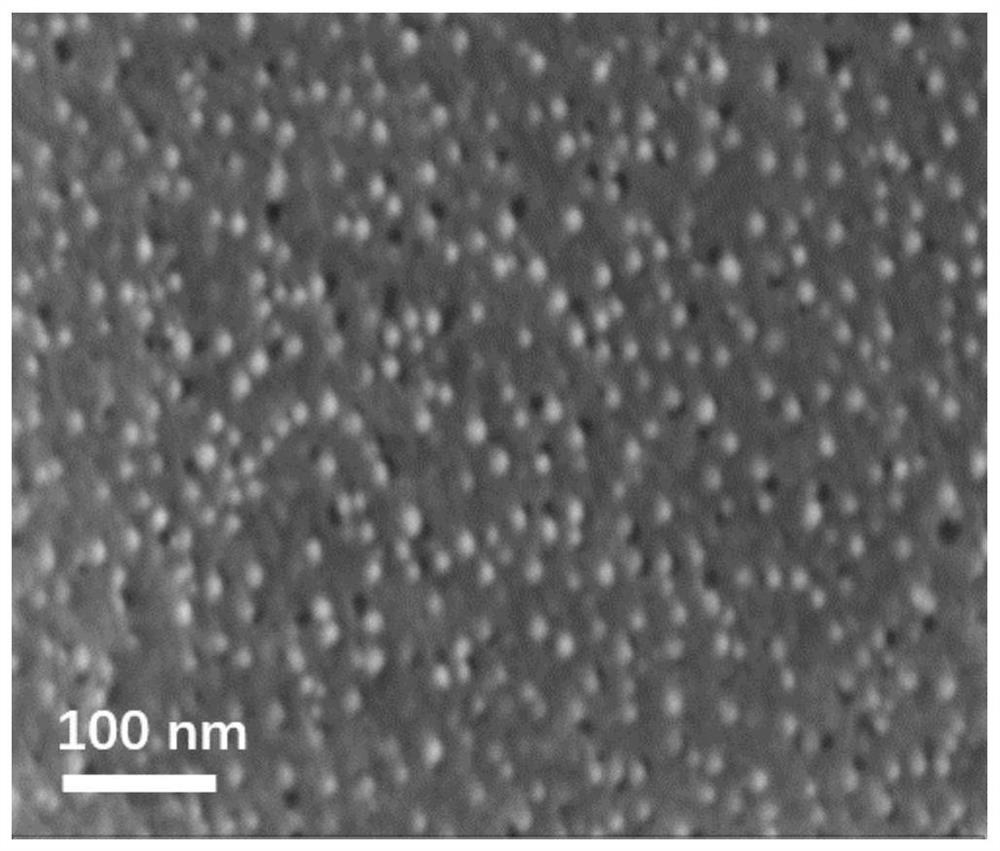
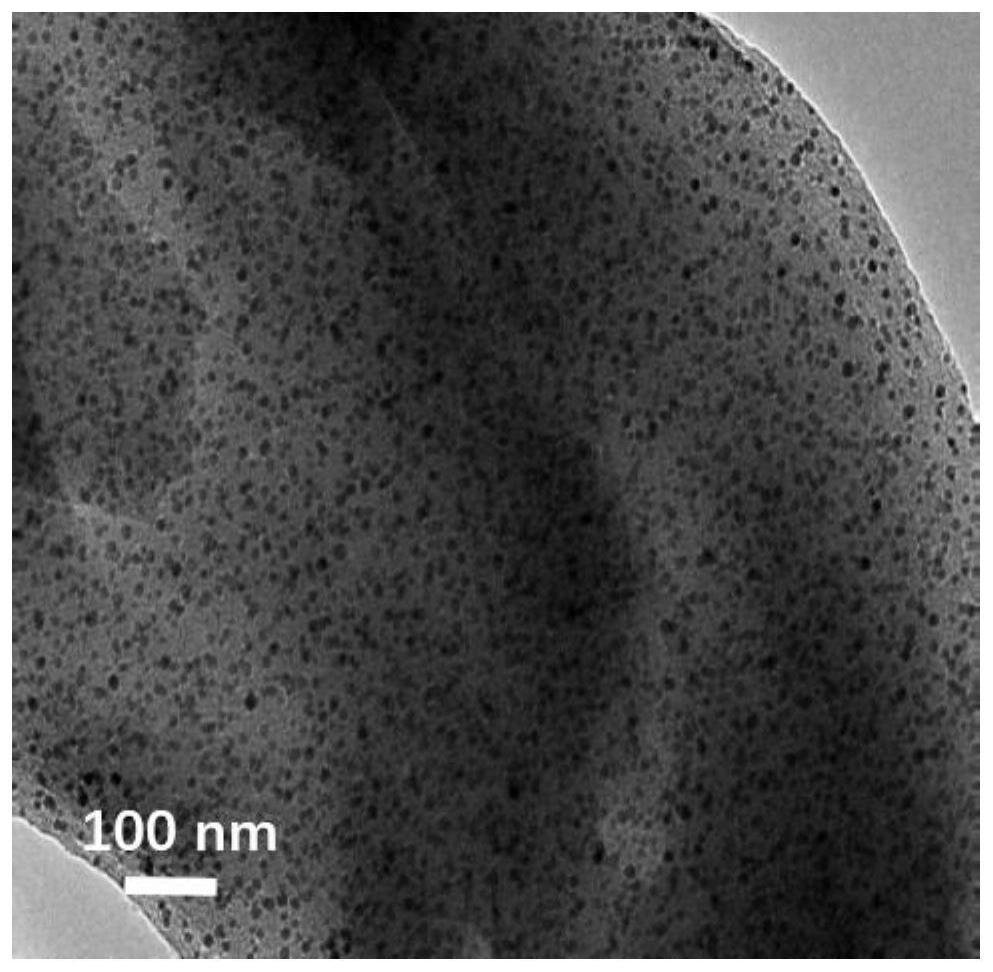
[0029] Embodiment 1: the preparation of catalyst Ni / ACP
[0030] 1. Preparation of activated carbon carrier (ACP) from cellulose
[0031] First, 18 g of cellulose powder was placed in a high-temperature tube furnace under a nitrogen atmosphere with a flow rate of 200 mL / min, heated from room temperature to 650 °C at a heating rate of 10 °C / min, and carbonized at 650 °C for 2 h. to prepare coke. After completion of carbonization, the obtained coke was ground into powder and mixed thoroughly with potassium hydroxide (KOH), the mass ratio of KOH to coke was 3:1, and the mixture was put into a tube furnace with a nitrogen atmosphere at a flow rate of 200 mL / min In the process, heating from room temperature to 700°C at a heating rate of 10°C / min, and activation treatment at 700°C for 2h. After the activation is completed, wash the product with the prepared 2mol / L dilute hydrochloric acid solution, and then continue washing with deionized water until the filtrate is neutral. Fina...
Embodiment 2
[0047] Embodiment 2: Application of catalytic hydrogenation of diphenyl ether
[0048] All catalytic reactions were performed in a 100 mL stainless steel autoclave.
[0049] The substrate diphenyl ether (100 mg), catalyst (50 mg) and isopropanol (20 mL) were placed in the reactor. After sealing, the residual air was removed by bubbling hydrogen 3 times. Subsequently, the reactor was pressurized to the desired pressure (1.0 MPa) with hydrogen at room temperature. The temperature was then raised to the desired reaction temperature (140° C.) and maintained for a certain time (120 min) at a vigorous stirring speed of 800 rpm. After the experiment, the reaction system was naturally cooled to room temperature and the pressure was released. The reaction mixture was filtered to remove the catalyst, and the obtained organic phase was analyzed by mass spectrometry (GC-MS) and gas phase (GC).
[0050] Table 2 The hydrogenation catalytic performance of different catalysts to diphenyl ...
Embodiment 3
[0053] Embodiment 3: the influence of reaction temperature on diphenyl ether conversion
[0054] Reaction procedure is the same as embodiment 2, and difference is reaction conditions: 100mg diphenyl ether, 20mL Virahol, 50mg Ni / ACP catalyst, 1MPa H 2 , 2h.
[0055] Depend on Figure 5 It can be seen that the conversion rate of diphenyl ether catalyzed by Ni / ACP catalyst increases gradually with the increase of reaction temperature, and only at 160 °C, the conversion rate reaches the maximum value of 100%, and then remains unchanged. At a lower reaction temperature, the product distribution is wider, and the yield of cyclohexane and cyclohexanol in the product increases gradually with the increase of the reaction temperature. At 180 ° C, a higher yield of cyclohexane and cyclohexanol is obtained. Cyclohexanol.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Average particle size | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
| Specific surface area | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com