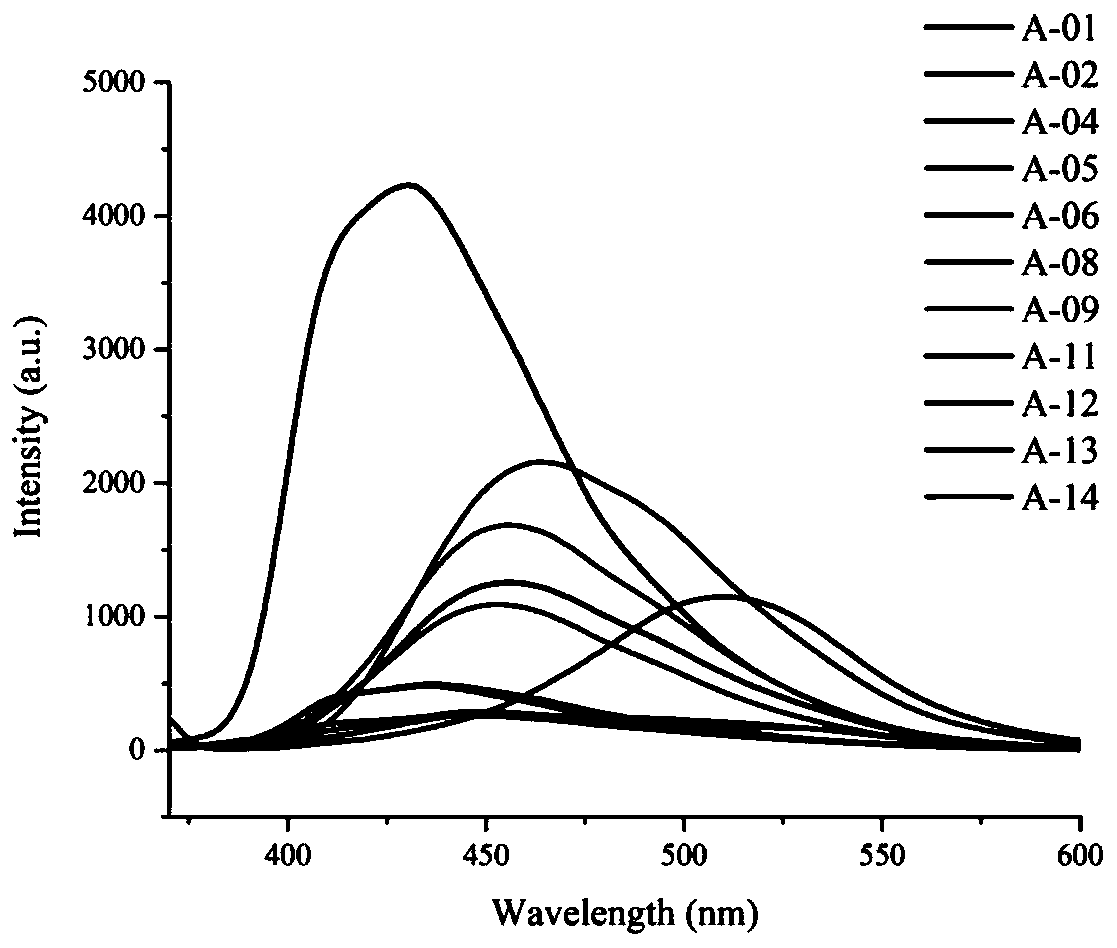
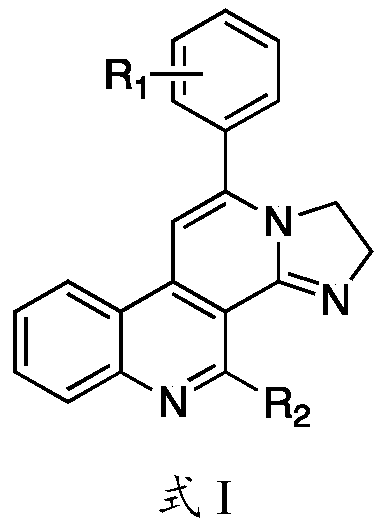
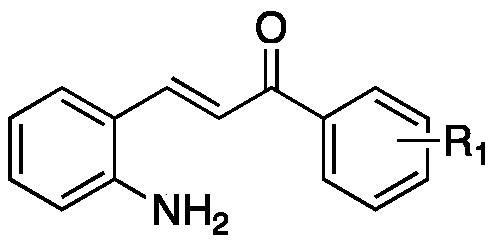
Imidazaphenanthridine compound as well as preparation method and application thereof
An imidazo, compound technology, applied in the field of compound synthesis, can solve the problems of low substrate diversity and low yield, and achieve the effects of wide application range, high reaction efficiency and mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042] Example 1 4,11-diphenyl-1,2-dihydrobenzo[f]imidazo[2,1-α][2,7]quinoline
[0043]
[0044] 3-(2-Aminophenyl)-1-phenylprop-2-en-1-one (0.6mmol, 133.8mg) and 2-(imidazolidine-2-ylidene)-1-phenylethane -1-ketone (0.5mmol, 94mg) was added to 1mL of glacial acetic acid. After the addition was complete, it was reacted at room temperature for 24h, and observed by spotting. After the reaction was completed, NaOH (1M) was added to the reaction solution for neutralization, and then extracted through the column to obtain a yellow solid.
[0045] Yellow solid, m.p.>250℃, 1 H NMR (500MHz, CDCl 3 )δ8.40(d, J=8.0Hz, 1H), 8.24(d, J=8.5Hz, 1H), 7.98–7.93(m, 1H), 7.80–7.72(m, 5H), 7.65–7.59(m ,6H),4.64–4.57(m,2H),4.22–4.15(m,2H). 13 C NMR (125MHz, CDCl 3 )δ159.3, 156.3, 149.4, 147.3, 142.9, 142.3, 135.5, 130.9, 130.3, 129.9, 129.2, 129.0, 128.1, 127.9, 126.6, 123.3, 121.8, 111.5, 98.3, 53.0, 49.1: HRMS (ESI). z calcd for (C 26 h 20 N 3 +H) + :374.1657; found: 374.1660.
Embodiment 2
[0046] Example 2 11-phenyl-4-(p-tolyl)-1,2-dihydrobenzo[f]imidazo[2,1-α][2,7]quinoline
[0047]
[0048] The synthetic steps are the same as in Example 1, except that 2-(imidazolidine-2-ylidene)-1-phenylethane-1-ketone is replaced by 2-(imidazolidine-2-ylidene)-1-(p-toluene base) ethyl-1-one to give a yellow solid.
[0049] Yelbow solid, m.p.234.1-235℃, 1 H NMR (500MHz, Chloroform-d) δ8.16(d, J=8.5Hz, 1H), 8.11(d, J=8.0Hz, 1H), 7.72(m, 1H), 7.60(d, J=8.0Hz ,2H),7.55(m,2H),7.53–7.50(m,3H),7.24(d,J=7.5Hz,2H),6.62(s,1H),3.87–3.76(m,4H),2.42( s,3H). 13C NMR (125MHz, DMSO) δ158.6, 154.9, 149.7, 146.6, 142.4, 139.6, 136.5, 135.1, 130.9, 129.7, 129.3, 129.0, 128.7, 128.0, 127.6, 126.6, 124.3, 121.3, 110.8, 2 ,21.0.HRMS(ESI):m / z calcd for(C 27 h 22 N 3 +H) + :388.1814; found: 388.1822.
Embodiment 3
[0050] Example 3 4-(4-chlorophenyl)-11-phenyl-1,2-dihydrobenzo[f]imidazo[2,1-α][2,7]quinoline
[0051]
[0052] The synthesis steps are the same as in Example 1, except that 2-(imidazolidine-2-ylidene)-1-phenylethane-1-one is replaced by 1-(4-chlorophenyl)-2-(imidazolidine-2 -ylidene)ethan-1-one to give a yellow solid.
[0053] Yellow solid, m.p.>250℃, 1 H NMR (500MHz, CDCl 3 )δ8.69(s,2H),8.15(m,2H),7.77(s,1H),7.62–7.48(m,8H),6.62(s,1H),3.80(s,4H). 13 C NMR (125MHz, CDCl 3 )δ156.9, 155.8, 150.4, 149.9, 149.3, 147.4, 143.0, 135.6, 131.1, 130.5, 130.0, 129.0, 127.9, 127.2, 127.1, 124.0, 123.4, 122.2, 111.5, 97.4: HR1.4, MS m / z calcd for (C 26 h 19 ClN 3 +H) + :408.1268; found: 408.1270.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com