Polymerization inhibitor and preparation method and application thereof
A technology of polymerization inhibitor and initiator, which is applied in the preparation of carboxylate, chemical instruments and methods, preparation of organic compounds, etc. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
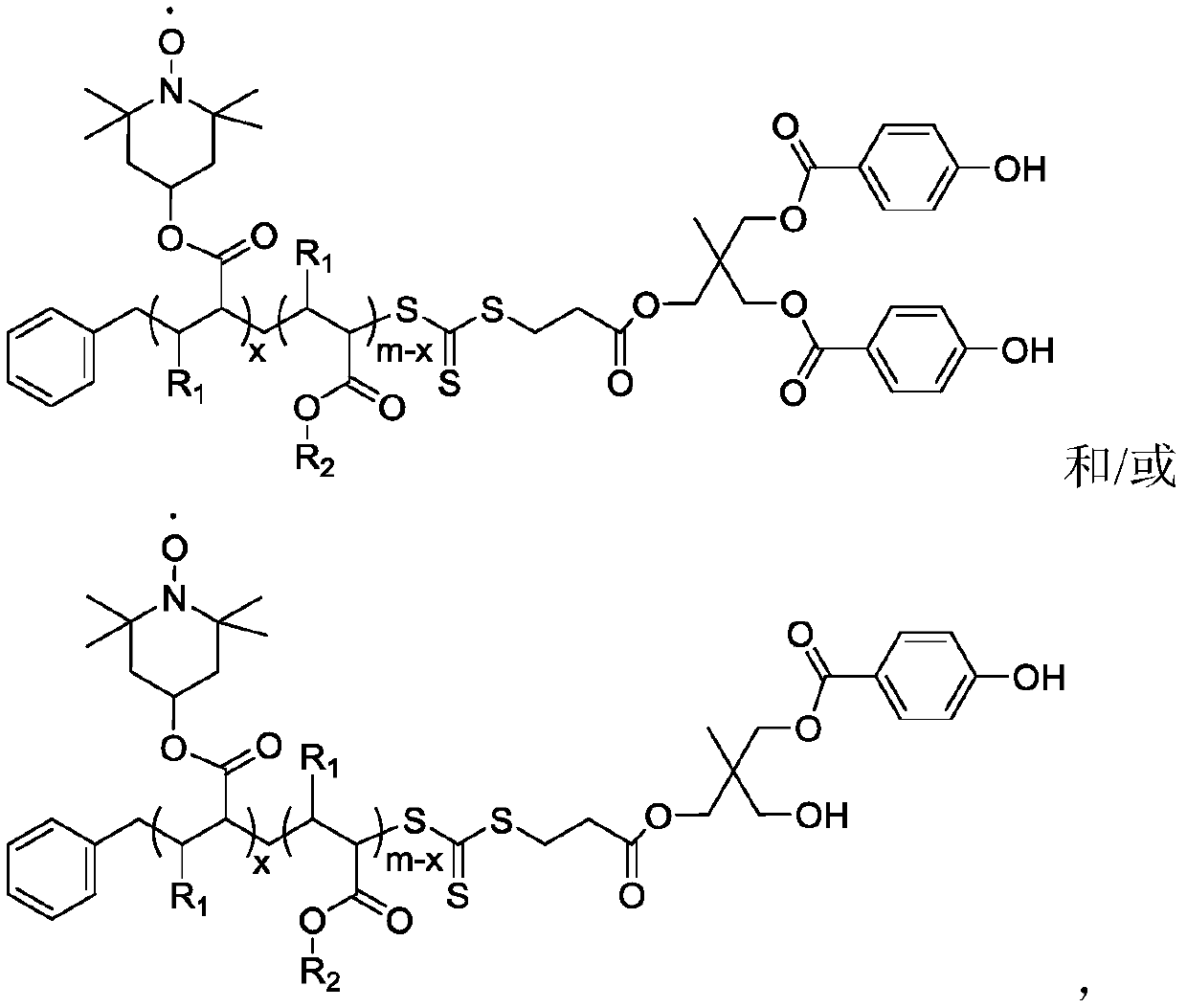
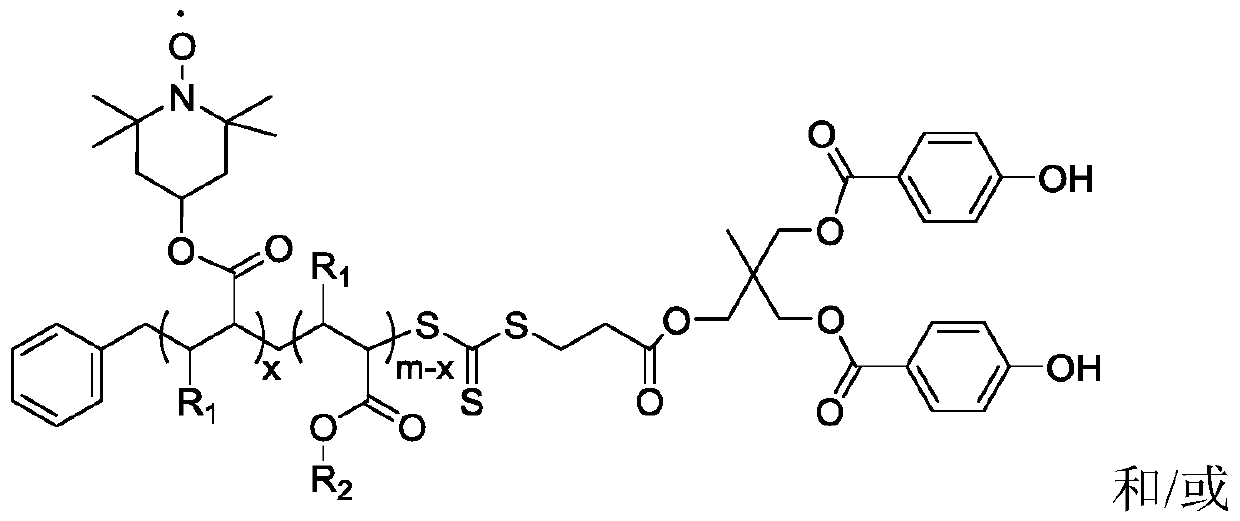
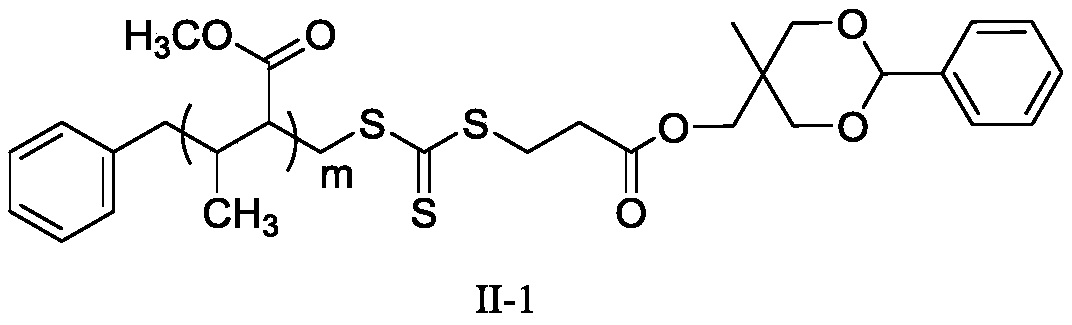
[0063] (1) Dissolve MHPD and benzyl trithiocarbonate propionic acid in dichloromethane, add catalyst 4-dimethylaminopyridine and N, N-dicyclohexylcarbodiimide, wherein benzyl trithio The molar ratio of carbonate-based propionic acid and MHPD is 1:1, the molar ratio of 4-dimethylaminopyridine and MHPD is 0.1:1, and the molar ratio of N,N-dicyclohexylcarbodiimide and MHPD is 1: 1. Under the condition of 20°C, react for 24 hours, filter after the reaction, concentrate by rotary evaporation, and precipitate with diethyl ether twice to obtain a light yellow solid, which is dried in vacuum to obtain the compound of formula I; Infrared analysis is carried out to the compound of formula I obtained, and 1732cm appears -1 ester bond peaks.
[0064] (2) Dissolve the compound of formula I and methyl methacrylate in N,N-dimethylformamide, and react under the initiation of 2,2'-azobisisobutyronitrile, wherein the initiator and formula I The molar ratio of the compound is 0.4:1, and the m...
Embodiment 2
[0073] (1) Dissolve MHPD and benzyl trithiocarbonate propionic acid in chloroform, add catalyst 4-dimethylaminopyridine and N, N-dicyclohexylcarbodiimide, wherein benzyl trithio The mol ratio of carbonate group propionic acid and MHPD is 3:1, the mol ratio of 4-dimethylaminopyridine and MHPD is 0.3:1, and the mol ratio of N,N-dicyclohexylcarbodiimide and MHPD is 3: 1. Under the condition of 50°C, react for 72 hours, filter after the reaction, concentrate by rotary evaporation, and precipitate with diethyl ether twice to obtain a light yellow solid, which is dried in vacuum to obtain the compound of formula I; Infrared analysis is carried out to the compound of formula I obtained, and 1737cm appears -1 ester bond peaks.
[0074] (2) Dissolving the compound of formula I and methyl acrylate in N,N-dimethylacetamide, and reacting under the initiation of 2,2'-azobisisoheptanonitrile, wherein the initiator and the compound of formula I The molar ratio is 0.6:1, the molar ratio of...
Embodiment 3
[0084] (1) MHPD and benzyl trithiocarbonate propionic acid are dissolved in the solvent chloroform, add catalyst 4-dimethylaminopyridine and N, N-dicyclohexylcarbodiimide, wherein benzyl trisulfide The molar ratio of carbonate group propionic acid and MHPD is 2:1, the molar ratio of 4-dimethylaminopyridine and MHPD is 0.2:1, and the molar ratio of N,N-dicyclohexylcarbodiimide and MHPD is 2 : 1, under the condition of 25°C, react for 48h, filter after the reaction, concentrate by rotary evaporation, and precipitate with solvent diethyl ether twice to obtain a light yellow solid, which is dried in vacuo to obtain the compound of formula I; Infrared analysis is carried out to the compound of formula I obtained, and 1725cm appears -1 ester bond peaks.
[0085] (2) Dissolve the compound of formula I and ethyl methacrylate in N,N-dimethylacetamide, and react under the initiation of 2,2'-azobisisobutyronitrile, wherein the initiator and formula I The molar ratio of the compound is...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com