Method for producing high-purity (R)-(-)-3-hydroxybutyric acid by adopting enzymatic method
A hydroxybutyric acid, high-purity technology, applied in the field of bioengineering, can solve the problems of simple process and inability to achieve efficient production of 3-hydroxybutyric acid monomer, and achieve high chemical purity, single optical activity, and high purity.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] Embodiment 1: Activation seed solution
[0036] The bacterial strain Diaphorobacterpolyhydroxybutyrativorans preserved in the lyophilized tube in Example 1 was inoculated in LB slant medium (yeast powder 5g / L, peptone 10g / L, sodium chloride 10g / L, agar 20g / L), 35°C, static Cultured for 12h. Pick a single colony of EMD bacteria, inoculate it in LB liquid medium (yeast powder 5g / L, peptone 10g / L, NaCl 10g / L), culture at 35°C, 180rpm for 12h with shaking, and prepare a first-grade seed solution. According to the inoculation amount of 10%, inoculate the primary seed solution in the enzyme production medium (PHB 1g / L, KNO 3 2g / L, KH 2 PO 4 1g / L, Na 2 HPO 4 3g / L, (NH 4 ) 2 SO 4 1g / L, MgCl 2 ·6H 2 O 0.2g / L, NaCl 0.2g / L, yeast powder 0.15g / L, CaCl 2 0.036g / L, ferric ammonium citrate 0.01g / L), 35°C, 180rpm shaking culture for 12h, to prepare secondary seed liquid.
Embodiment 2-1
[0041] Example 2-1: Enzyme Production by Fermentation: Enzyme Production Activity of PHB as the Only Carbon Source and Inducer
[0042] The secondary seed liquid of Example 1 was cultured and fermented with a 250mL shake flask, the filling volume was 100mL, and the medium components were: PHB 1g / L, KNO 3 2g / L, KH 2 PO 4 1g / L, Na 2 HPO 4 3g / L, (NH 4 ) 2 SO 4 1g / L, MgCl 2 ·6H 2 O 0.01g / L, NaCl 0.2g / L, yeast powder 0.15g / L, CaCl 2 0.02g / L, ferric ammonium citrate 0.01g / L. The culture temperature is 35°C, the pH value is about 7.2, the rotation speed is 180 rpm, and the culture time is 18 hours. The fermentation time was 18 hours, the cells were removed by centrifugation, and the supernatant was taken to measure the enzyme activity. The total enzyme activity was 210U.
Embodiment 2-2 to 2-4
[0043] Embodiment 2-2 to 2-4: Enzyme production by fermentation
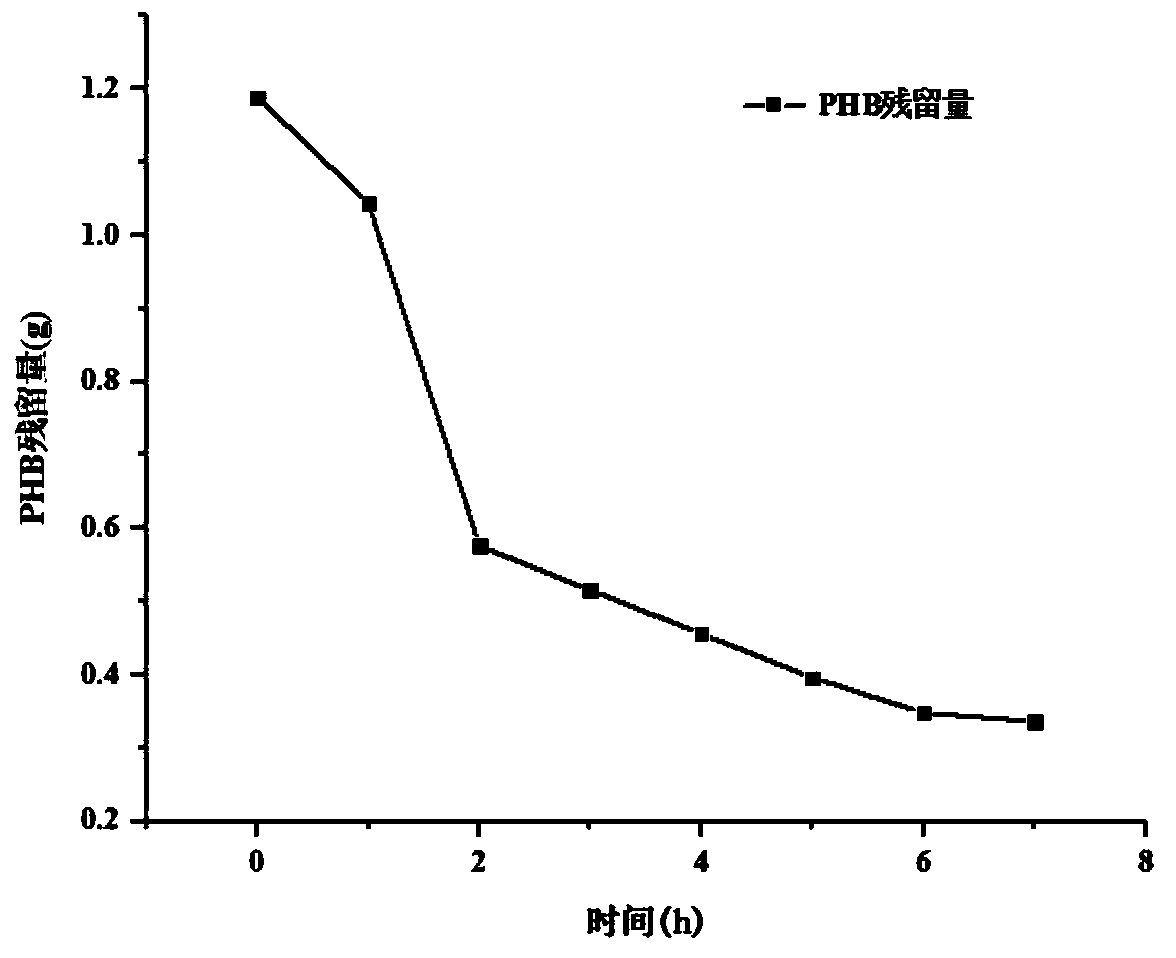
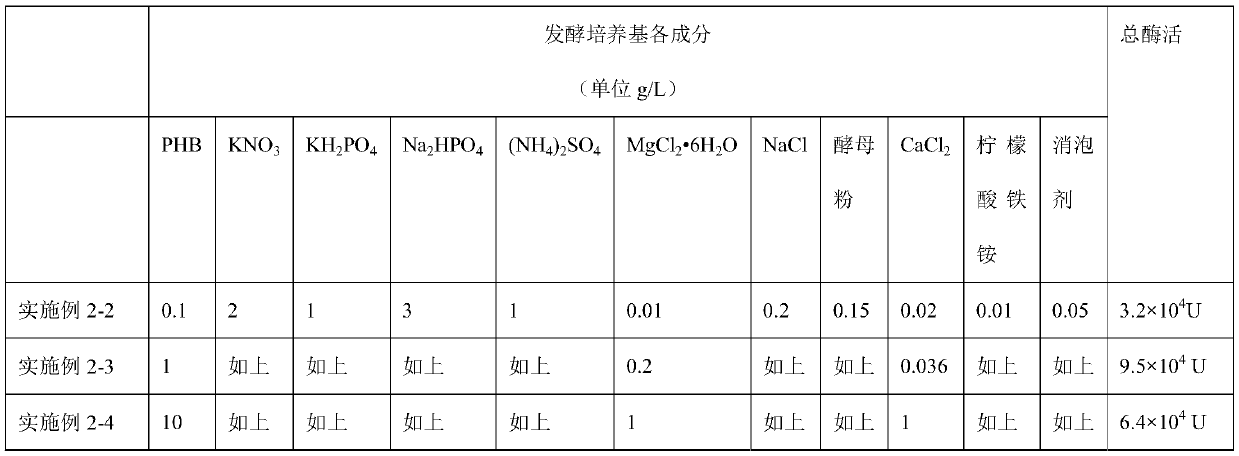
[0044] The secondary seed solution of Example 1 of 500 mL was inoculated in a 7 L fermenter, and the filling volume was 5 L. The components of the fermentation medium are listed in Table 1. The culture temperature is 35°C, the pH value is about 7.2, the dissolved oxygen constant is controlled at 20-25%, and the ventilation rate and stirring speed are flexibly adjusted according to the dissolved oxygen constant. Feed PHB was fed, and the concentration of PHB was controlled at 0.5g / L throughout the fermentation enzyme production process. The total fermentation time is 18h.
[0045] Table 1 embodiment 2-2 to 2-4 adopts the fermentation medium total enzyme activity of different content components
[0046]
PUM
| Property | Measurement | Unit |
|---|---|---|
| specific rotation | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com