Primer, probe and reagent kit for quick and quantitative detection by AR-V7 fluorescent qRT-PCR method
An AR-V7, detection reagent technology, applied in the determination/inspection of microorganisms, DNA/RNA fragments, recombinant DNA technology, etc., can solve the problem of not being able to fully reflect the disease state of patients, requiring high freshness of whole blood samples, The high heterogeneity of tumor cells can reduce the risk of contamination or errors, ensure reliable detection results, and shorten the experimental period.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0061] Example 1 Extraction of exosomes
[0062] Materials: 8 cases of EDTA tubes were used to collect plasma samples, and exosomes were prepared using automatic exosome extraction reagents.
[0063] Experimental steps:
[0064] 1. Plasma sample pretreatment
[0065] Take a plasma sample of 16.000 g, centrifuge at 4 °C for 10 min, and take 1 mL of the supernatant for use;
[0066] 2. The formula of exosome automatic extraction reagent is as follows:
[0067] (1) Magnetic bead suspension, mainly carboxyl magnetic beads coated with 1 mg / mL CD63 antibody;
[0068] (2) Washing solution, mainly 0.01 M phosphate buffer solution of 0.02 mg / mL bovine serum albumin;
[0069] (3) Eluent, mainly 0.01 M phosphate buffer;
[0070] 3. The parameter setting of exosome automatic extraction equipment is as follows:
[0071]
[0072] 4. The specific operation process is as follows:
[0073] (1) Take a 96-well deep-well plate, add 1 mL of plasma from 8 samples in sequence to well 1, an...
Embodiment 2
[0080] Example 2 Extraction of exosome nucleic acid
[0081] Materials: Take 100 μL of phosphate buffer solution containing exosomes and magnetic beads prepared in Example 1, use the exosome nucleic acid extraction reagent of the present invention, and use an automatic nucleic acid purifier to prepare exosome RNA;
[0082] experiment process:
[0083] The exosome nucleic acid extraction reagent provided by the present invention uses the KY-32A automated nucleic acid extraction workstation of Kangyu Biotechnology to extract exosome RNA, and the reagent addition method is as follows:
[0084] Add 10 μL of exosome resuspension, 800 μL of solution I, and 10 μL of solution II to the 8 wells in the first column of the 96-deep well plate. Add 40 μL solution III to the 8 wells in column 2, add 300 μL washing solution A to the 8 wells in column 3, add 400 μL washing solution B to the 8 wells in column 4, and add 400 μL washing solution B to the 8 wells in column 5. Add 500 μL of wash...
Embodiment 3
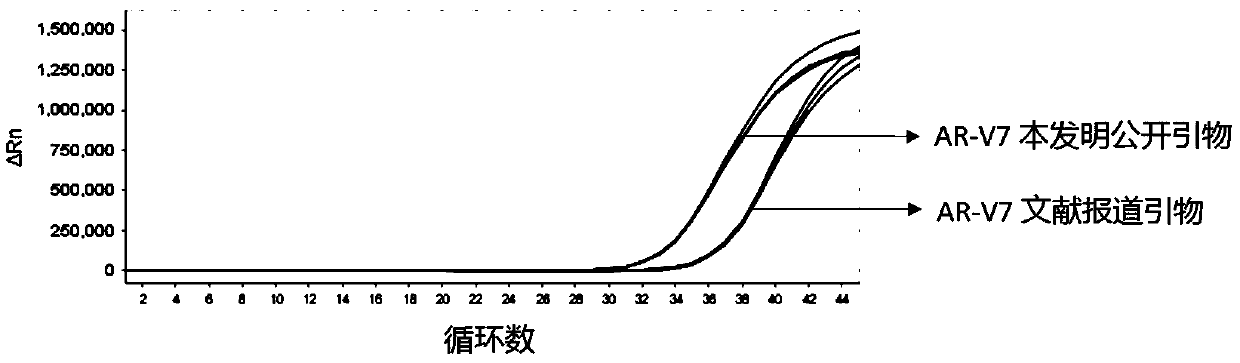
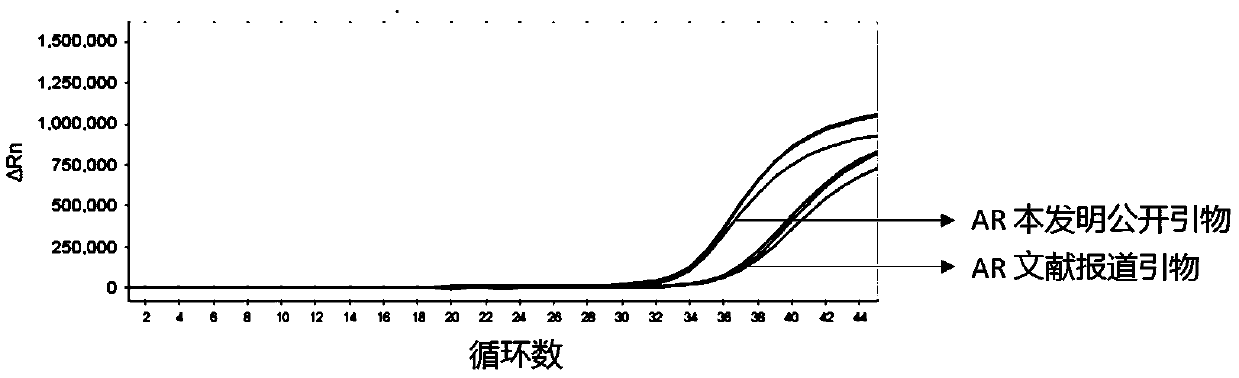
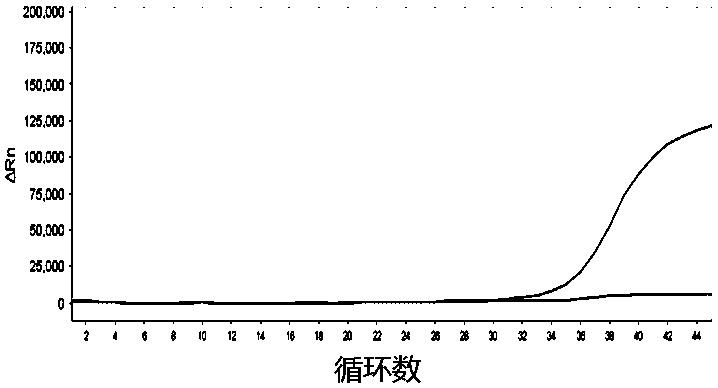
[0092] Example 3 Screening and optimization of AR-V7 primer pair and AR primer pair
[0093] The primer sequences for detecting AR-V7 and AR disclosed by the present invention are as follows:
[0094] AR-V7 forward primer: CAGGGATGACTCTGGGAGAAA;
[0095] AR-V7 reverse primer: AGTCAGCCTTTTCTTCAGGGTC;
[0096] AR forward primer: TGCTCAAGACGCTTCTACCAG;
[0097] AR reverse primer: AGTGAACTGATGCAGCTCTC;
[0098] The primer sequences for detecting AR-V7 and AR reported in the literature (AR-V7 and resistance to enzalutamide and abiraterone in prostate cancer. N Engl J Med 2014;371:1028-38) are as follows:
[0099] AR-FL forward primer: CAGCCTATTGCGAGAGAGCTG;
[0100] AR-FL reverse primer: GAAAGGATCTTGGGCACTTGC;
[0101] AR-V7 forward primer: CCATCTTGTCGTCTTCGGAAATGTTA;
[0102] AR-V7 reverse primer: TTTGAATGAGGCAAGTCAGCCTTTCT;
[0103] The comparison test of the primer pair was performed by using the exosomal RNA in the culture supernatant of 22RV1 cells by ultra-high speed c...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com