Method for preparing lidocaine
A technology for lidocaine and dimethylaniline, applied in the field of preparation of lidocaine, to achieve the effects of high product purity, simple post-processing, and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
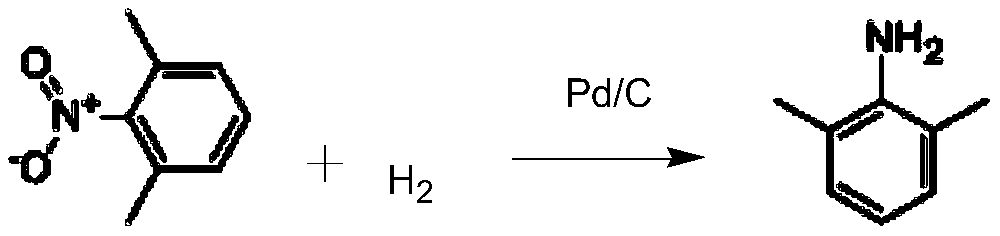
[0032] (1) Preparation of intermediate 2,6-dimethylaniline
[0033] Add 80g of 2,6-dimethylnitrobenzene, 5g of 5% Pd / C catalyst, and 160g of anhydrous methanol into a 500ml three-necked flask. After replacing with hydrogen, carry out hydrogenation reaction, filter after 20-25°C until the reaction is complete, recover Pd / C for recycling; the filtrate is distilled under reduced pressure, recover methanol for recycling, and obtain 59.2g of 2,6-dimethylaniline , yield 92.4%, HPLC purity 99.86%.
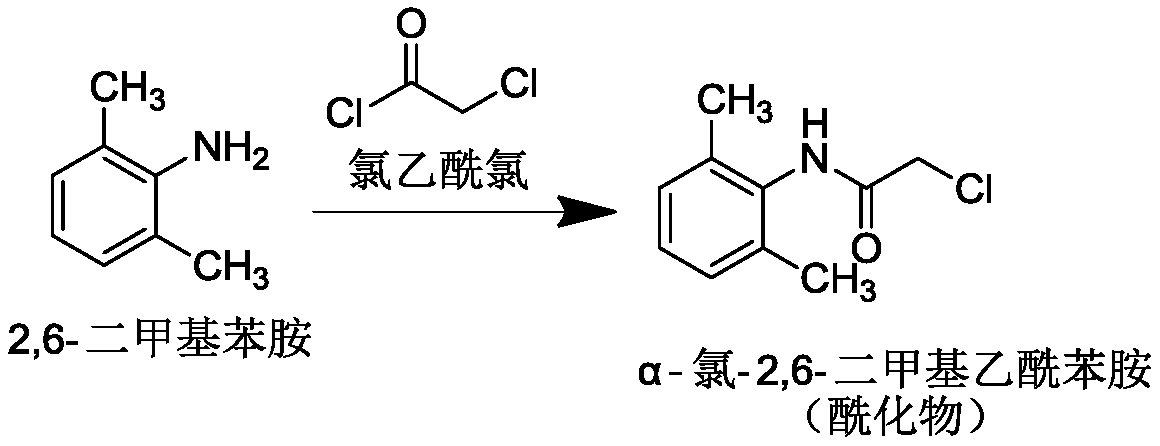
[0034] (2) Preparation of intermediate chloroacetyl-2,6-dimethylaniline
[0035] Weigh 48.4g of 2,6-dimethylaniline, 27.6g of potassium carbonate, and 275g of dichloromethane, and add them to the reaction flask in sequence, stir evenly, add 53.0g of chloroacetyl chloride dropwise, and react at room temperature for 1 hour. Dichloromethane was evaporated, washed with purified water and then dried to obtain 71.2 g of chloroacetyl-2,6-dimethylaniline with a yield of 91.0%.
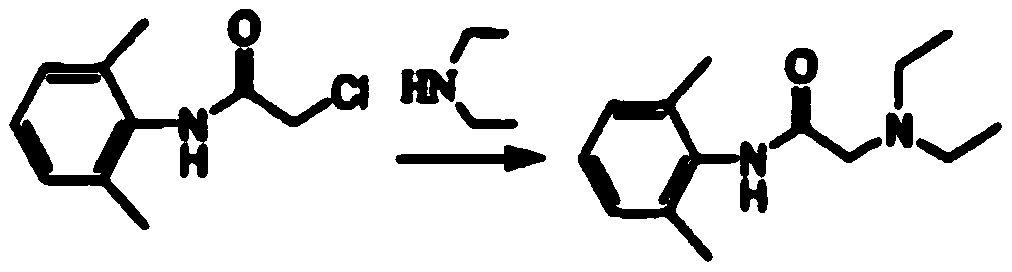
[0036] (3) pre...
Embodiment 2
[0039] (1) Preparation of intermediate 2,6-dimethylaniline
[0040] Add 60g of 2,6-dimethylnitrobenzene, 4g of 5% Pd / C catalyst, and 115g of anhydrous methanol into a 500ml three-necked flask. After replacing with hydrogen, carry out hydrogenation reaction, filter after 25-30°C until the reaction is complete, recover Pd / C for recycling; the filtrate is distilled under reduced pressure, recover methanol for recycling, and obtain 43.2g of 2,6-dimethylaniline , yield 89.9%, HPLC purity 99.57%.
[0041] (2) Preparation of intermediate chloroacetyl-2,6-dimethylaniline
[0042] Weigh 36.3g of 2,6-dimethylaniline, 20.7g of potassium carbonate, and 205g of dichloromethane, and add them to the reaction flask in sequence. After stirring evenly, add 40.0g of chloroacetyl chloride dropwise, and react at room temperature for 1 hour. Dichloromethane was evaporated, washed with purified water and then dried to obtain 52.9 g of chloroacetyl-2,6-dimethylaniline with a yield of 90.3%.
[004...
Embodiment 3
[0046] (1) Preparation of intermediate 2,6-dimethylaniline
[0047] Add 40g of 2,6-dimethylnitrobenzene, 2g of 5% Pd / C catalyst, and 80g of anhydrous methanol into a 500ml three-necked flask. After replacing with hydrogen, carry out hydrogenation reaction, filter after the reaction is complete at 20-30°C, recover Pd / C for recycling; the filtrate is distilled under reduced pressure, recover methanol for recycling, and obtain 29.3g of 2,6-dimethylaniline , yield 91.4%, HPLC purity 99.22%.
[0048] (2) Preparation of intermediate chloroacetyl-2,6-dimethylaniline
[0049] Weigh 24.2g of 2,6-dimethylaniline, 13.8g of potassium carbonate, and 136g of dichloromethane, and add them to the reaction flask in sequence, stir well, then add 26.5g of chloroacetyl chloride dropwise, and react at room temperature for 0.5h. Dichloromethane was evaporated, washed with purified water and then dried to obtain 34.9 g of chloroacetyl-2,6-dimethylaniline with a yield of 89.3%.
[0050](3) prepara...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com