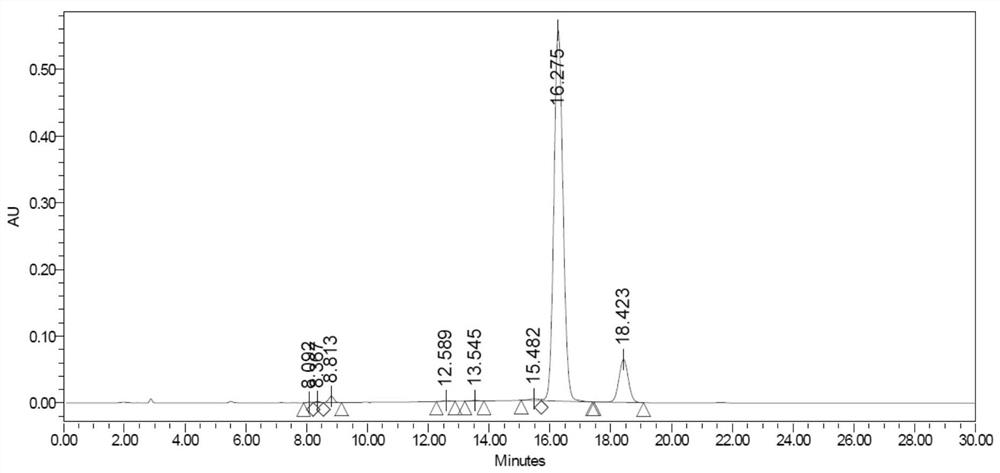
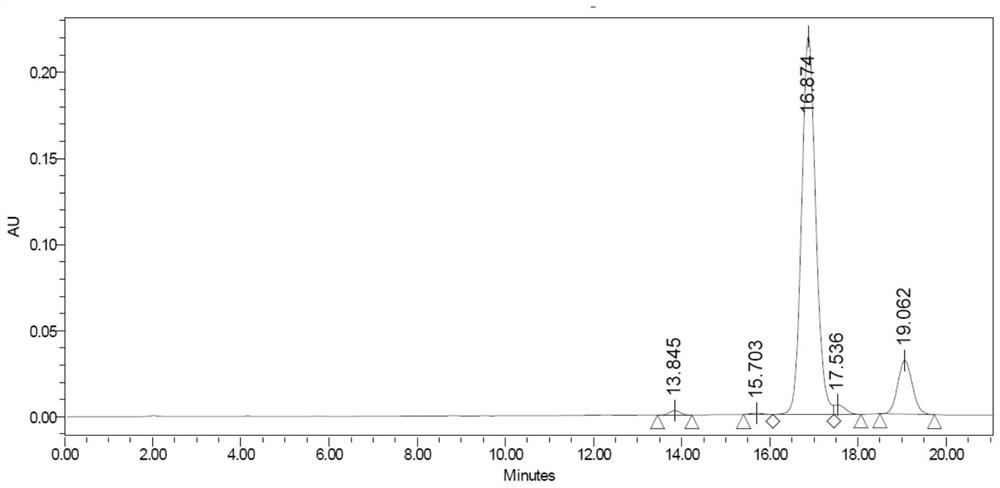
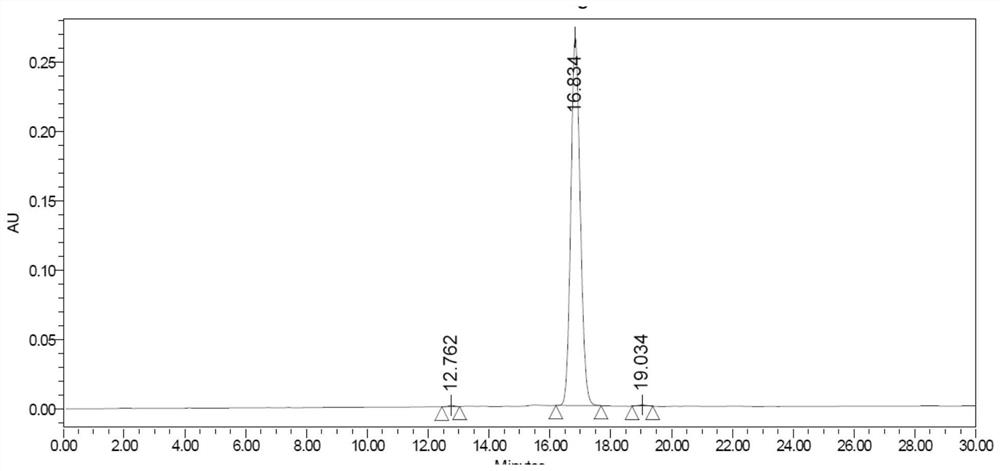
A kind of total liquid phase synthetic method of cinapultide
A cinaputide, liquid phase technology, applied in the field of total liquid phase synthesis of cinaputide, can solve the problems of low total yield, high cost, easy shrinkage, etc., and achieve the effect of reducing cycle time, simple method and reducing dosage
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0065] Embodiment 1, the synthesis of fully protected peptide 1
[0066] Preparation of Fmoc-Leu-ONb: Weigh 3534g (10mol) of Fmoc-Leu-OH and 1969g (11mol) of HONb and dissolve in 15L tetrahydrofuran, and stir in an ice-water bath. Weigh 2476g (12.0mol) of DCC, dissolve in 10L tetrahydrofuran, slowly add dropwise to the above solution, stir the reaction, and monitor the reaction with TLC. Suction filtration was performed after the reaction was completed, the reaction solution was concentrated to 8 L, and then 50 L of tertiary methyl ether was added to the concentrated solution, a large amount of white solid was precipitated, and the solution was left standing at -20°C for 4 hours. Suction filtration, the solid was dissolved in 5L of ethyl acetate, 50L of tertiary methyl ether was added for crystallization, suction filtration, and the solid was dried in vacuum to obtain 4837g of Fmoc-Leu-ONb with a purity greater than 99% and a yield of 94.0%.
[0067] Preparation of Fmoc-Leu-L...
Embodiment 2
[0073] Embodiment 2, the synthesis of fully protected peptide 2
[0074] Preparation of H-Leu-Leu-Leu-Leu-Lys(Boc)-OH: Weigh Fmoc-Leu-Leu-Leu-Leu-Lys(Boc)-OH1470g (1.6mmol), stir and dissolve with 8L dichloromethane , and then weighed 427g AlCl 3 (3.2mmol) and 242g N-methylmorpholine (2.4mol), were added to the reaction solution and stirred for 3 hours, and the reaction was monitored by TLC. The reaction solution was rotary evaporated to 1.6L, added to 16L of tertiary methyl ether for precipitation, the solid precipitate was collected by centrifugation, and after vacuum drying, 1029g of H-Leu-Leu-Leu-Leu-Lys(Boc)-OH was obtained, with a purity greater than 98.5%. Yield 92%.
Embodiment 3
[0075] Embodiment 3, the synthesis of fully protected peptide 3
[0076]Preparation of Fmoc-Leu-Leu-Leu-Leu-Lys(Boc)-ONb: Weigh Fmoc-Leu-Leu-Leu-Leu-Lys(Boc)-OH1470g (1.6mmol) and HONb 322g (1.8mol) in 4L tetrahydrofuran, stirring in an ice-water bath. Weigh 396g (1.9mol) of DCC, dissolve it in 4L tetrahydrofuran, slowly add it dropwise to the above solution, stir the reaction, and monitor the reaction by TLC. Suction filtration was performed after the reaction was completed, the reaction solution was concentrated to 3 L, 20 L of tertiary methyl ether was added to the concentrated solution, a large amount of white solid was precipitated, and the solution was left standing at -20°C for 4 hours. After standing still, filter with suction, dissolve the solid with 3L of ethyl acetate, add 25L of tertiary methyl ether, crystallize, filter with suction, and dry the filter cake in vacuum to obtain 1538g of Fmoc-Leu-Leu-Leu-Leu-Lys(Boc)-ONb , the purity is greater than 98.5%, and the...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com