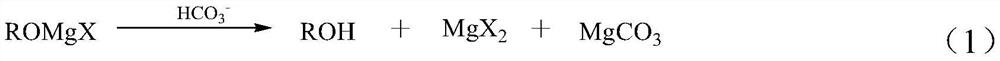Process for preparing alcohols by hydrolysis of metal-organic products
A metal-organic and product technology, applied in the field of metal-organic product post-processing technology, can solve problems such as product purity decline and hydrolysis process yield decline, and achieve the effects of avoiding halogenated side reactions and improving hydrolysis yield and product content.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
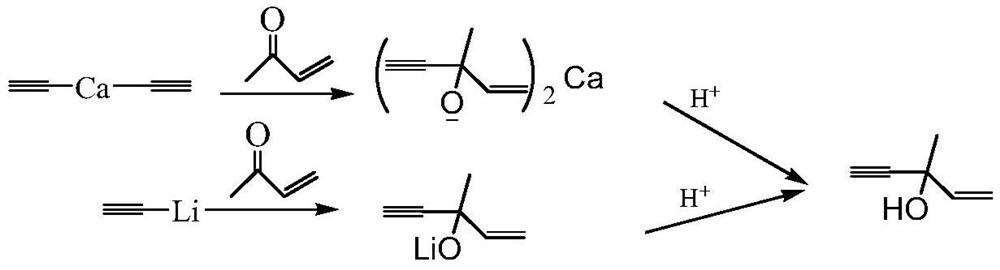
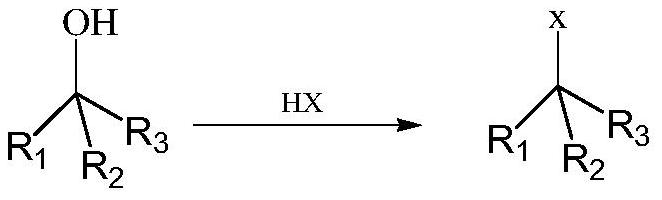
[0047] The metal-organic product obtained by the Grignard reaction is referred to as the magnesium dichloride condensate for short, and the structure is as follows:
[0048]
[0049] Add 500 mL of an aqueous solution containing 0.3 mol of magnesium bicarbonate into a four-port reactor, then dropwise add 300 mL of an ether solution containing 0.295 mol of magnesium dichloride condensate (this solution also contains 4% of 3-methyl-2-ene- 4-pentynyl alcohol (abbreviated as C6 alcohol, the same below)), the dripping is finished in about 20 minutes, and the hydrolysis reaction is stopped after continuing to stir for 60 minutes. The temperature of the hydrolysis process is controlled between 0 and 20°C. Filtration obtained 49.5 g of solid magnesium carbonate, and the oil-water mixture was separated to obtain 295 mL of ether solution of alcohol products and 490 mL of magnesium salt solution.
[0050] The diethyl ether solution of alcohol products is first reclaimed diethyl ether a...
Embodiment 2
[0059] The metal-organic product obtained by the Grignard reaction is referred to as dibromomagnesium condensate for short, and the structure is as follows:
[0060]
[0061] 500 mL of aqueous solution containing 0.3 mol of magnesium bicarbonate was added to the four-port reactor, and then 300 mL of methyl tetrahydrofuran solution containing 0.295 mol of dibromomagnesium condensate (containing 3% of 3-methyl-2-ene-4 -pentynyl alcohol (abbreviated as C6 alcohol, the same below)), the dripping is completed in about 100 minutes, and then the hydrolysis reaction is stopped after continuing to stir for 10 minutes, and the temperature of the hydrolysis process is controlled between 5 and 25°C. Then it was filtered to obtain 49.4 g of solid magnesium carbonate. The oil-water mixture was separated to obtain 290 mL of a dihydric alcohol solution in methyl tetrahydrofuran and 520 mL of a magnesium salt solution.
[0062] The methyl tetrahydrofuran solution of dibasic alcohol is firs...
Embodiment 3
[0071] The metal-organic product obtained by the ethynylation reaction of methyl ketene is bis(3-methyl-4-yn-1-penten-3-ol) calcium, with the following structure:
[0072]
[0073] Add 500 mL of an aqueous solution containing 0.3 mol of calcium bicarbonate to a four-port reactor, then dropwise add 300 mL of an isopropyl ether solution containing 0.299 mol of 3-methyl-4-yne-1-penten-3-yl calcium, for 60 min After dripping left and right, continue to stir for 30 minutes and then stop the hydrolysis reaction. The temperature of the hydrolysis process is controlled between 30 and 50°C. Centrifuge then to obtain solid calcium carbonate 59.6g. The oil-water mixture was separated to obtain 289 mL of 3-methyl-4-yn-1-penten-3-ol in isopropyl ether and 504 mL of calcium salt solution.
[0074] The isopropyl ether solution of 3-methyl-4-yne-1-penten-3-ol was recovered at 60-80°C under normal pressure, and then rectified to obtain 57.4g of the product, the content of which was detecte...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com