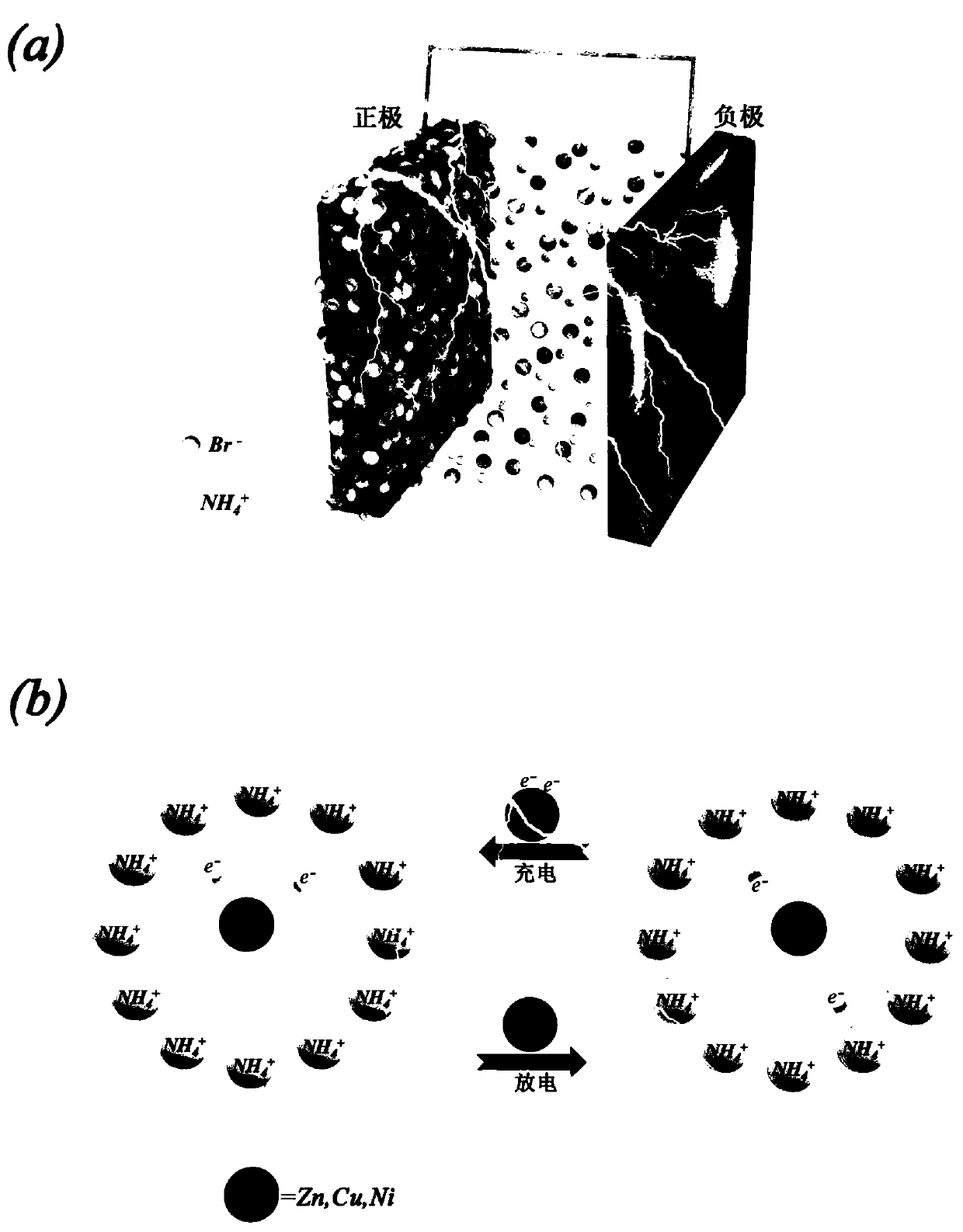
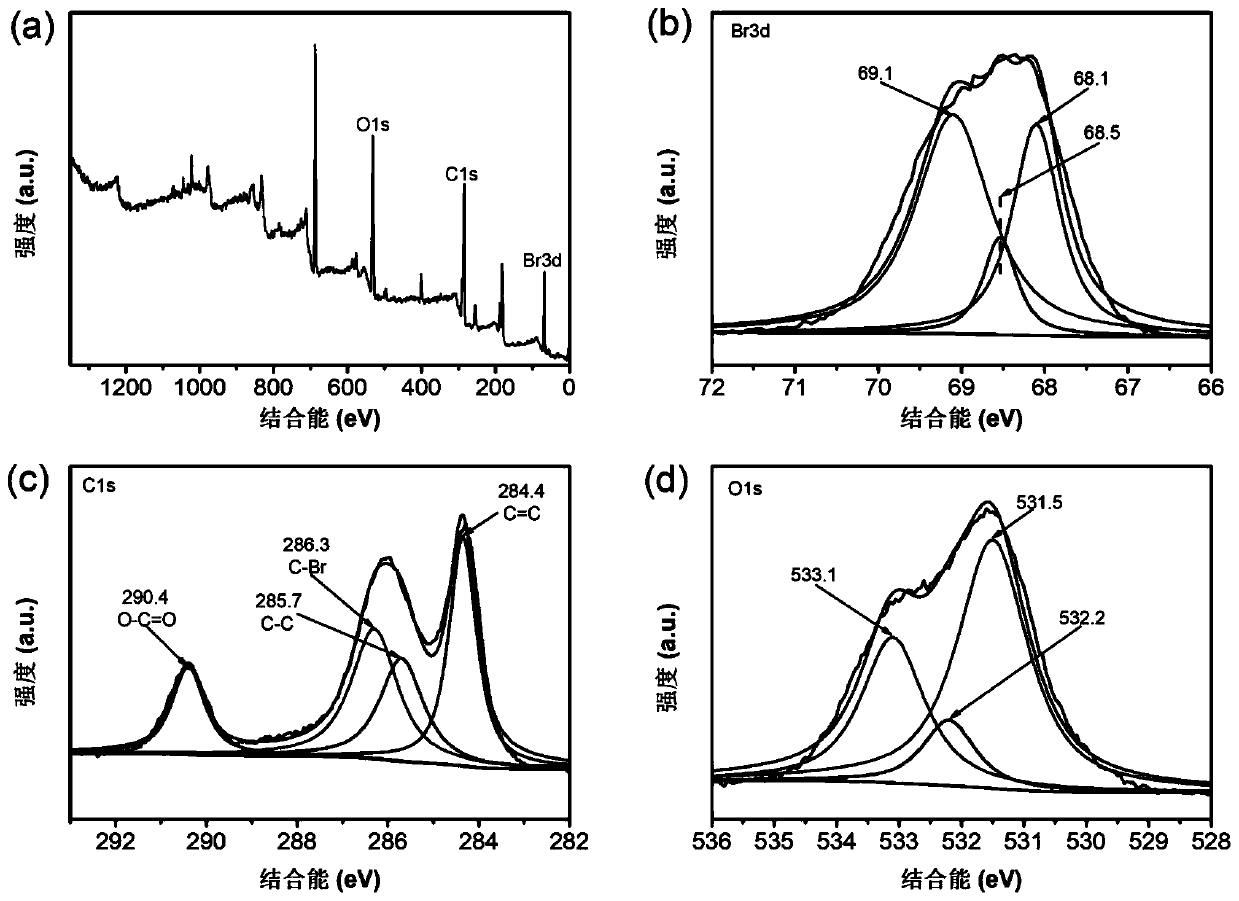
Bromine ion battery
A bromide ion and battery technology, applied in the field of new bromide ion batteries, can solve the problems of restricting development, poisonous bromine element, etc., and achieve the effect of improving safety, low price, and helping large-scale promotion
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
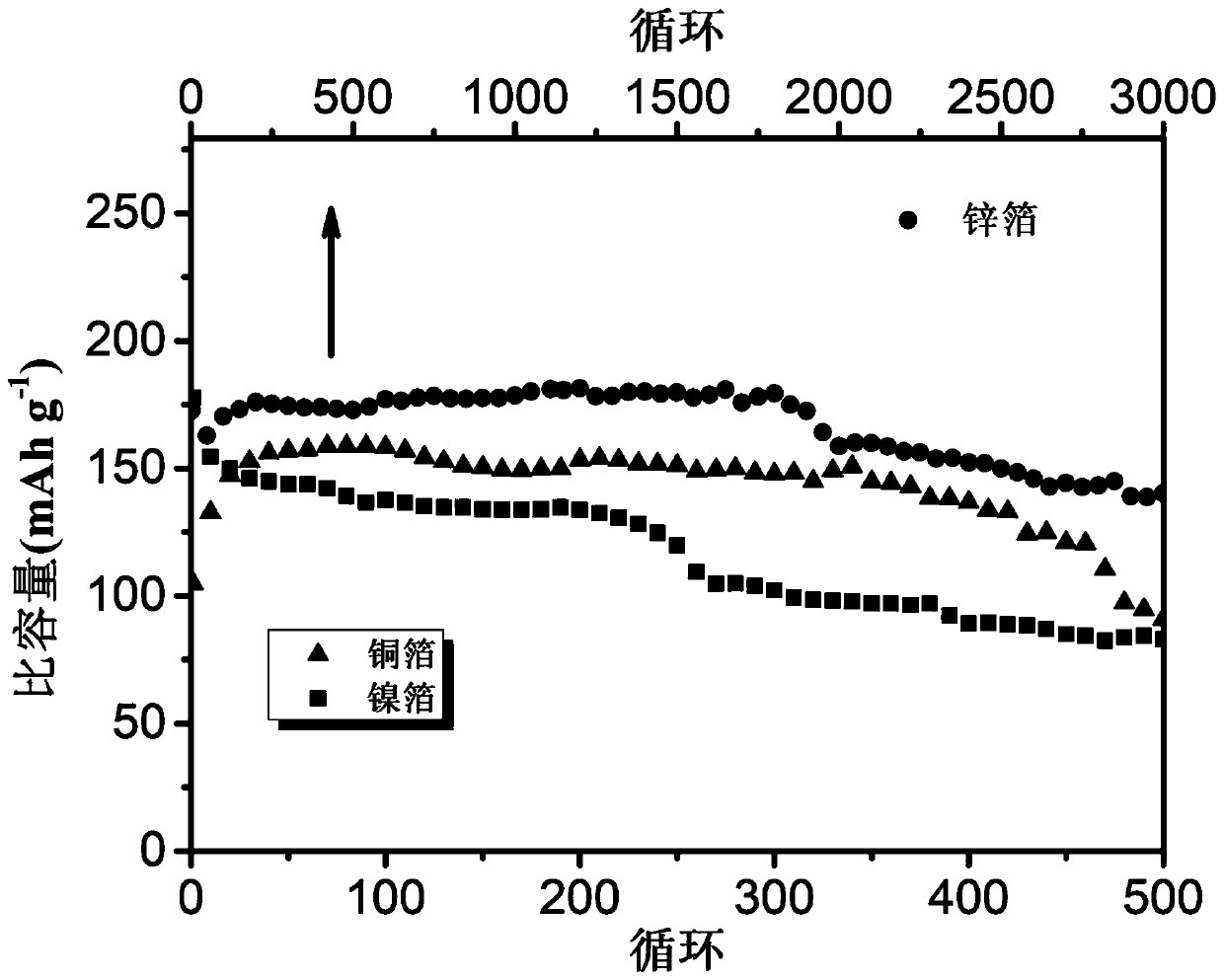
[0018] Put carbon black and binder (PVDF) in an agate grinding bowl with a mass ratio of 80:20, use N-methylpyrrolidone (NMP) as a solvent to grind and make slurry, and then smear the slurry on a 50 micron thick The area is 2.5cm*2.5cm on several stainless steel foils, and finally put the smeared sheet in a vacuum drying oven, and dry it at 80°C for 12 hours to obtain a working electrode sheet. Dissolve 1.96g of ammonium bromide in 10ml of ethylene glycol to prepare a 2mol / L electrolyte. Use 50 micron thick zinc foil as the negative electrode material of the bromide ion battery, assemble it into a pouch battery for performance testing, as shown in the attached image 3 As shown, under the charge and discharge current density of 2A / g, the capacity of the bromide ion battery still reaches 141mAh / g after 3000 cycles.
Embodiment 2
[0020] Put carbon black and binder (PVDF) in an agate grinding bowl with a mass ratio of 80:20, use N-methylpyrrolidone (NMP) as a solvent to grind and make slurry, and then smear the slurry on a 50 micron thick The area is 2.5cm*2.5cm on several stainless steel foils, and finally put the smeared sheet in a vacuum drying oven, and dry it at 80°C for 12 hours to obtain a working electrode sheet. Dissolve 1.96g of ammonium bromide in 10ml of ethylene glycol to prepare a 2mol / L electrolyte. Use copper foil with a thickness of 50 microns as the negative electrode material of bromide ion battery, and assemble it into a pouch battery for performance testing, as shown in the attached image 3 As shown, under the charge and discharge current density of 2A / g, the bromide ion battery has a capacity of 85mAh / g after 500 cycles.
Embodiment 3
[0022] Put carbon black and binder (PVDF) in an agate grinding bowl with a mass ratio of 80:20, use N-methylpyrrolidone (NMP) as a solvent to grind and make slurry, and then smear the slurry on a 50 micron thick The area is 2.5cm*2.5cm on several stainless steel foils, and finally put the smeared sheet in a vacuum drying oven, and dry it at 80°C for 12 hours to obtain a working electrode sheet. Dissolve 1.96g of ammonium bromide in 10ml of ethylene glycol to prepare a 2mol / L electrolyte. Use 50 micron thick nickel foil as the negative electrode material of the bromide ion battery, and assemble it into a pouch battery for performance testing, as shown in the attached image 3 As shown, under the charge and discharge current density of 2A / g, the bromide ion battery has a capacity of 85mAh / g after 500 cycles.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com