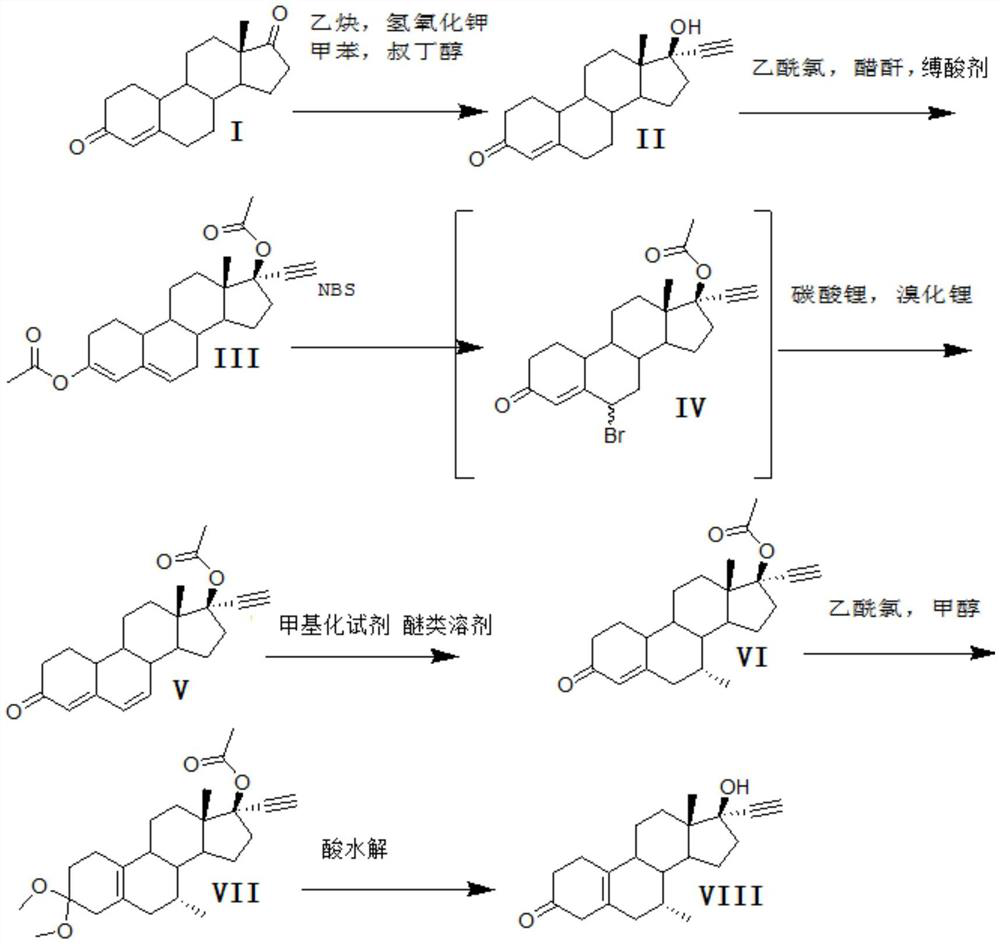
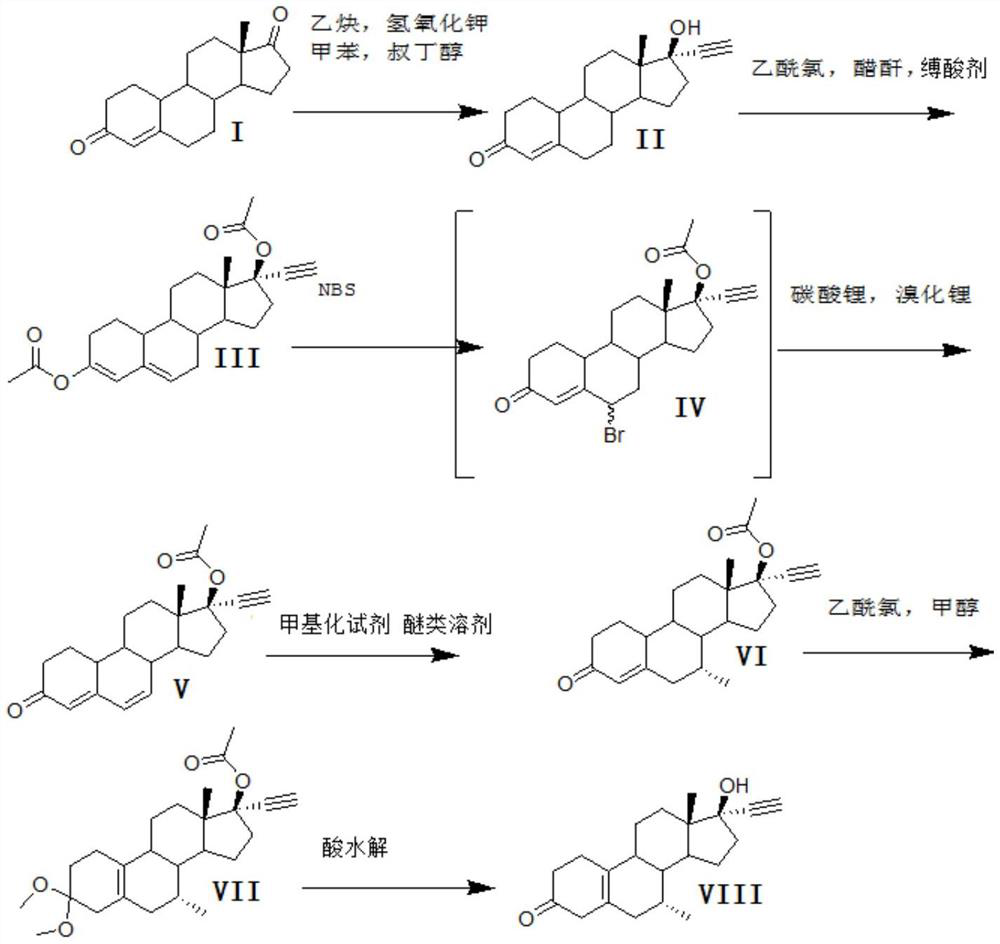
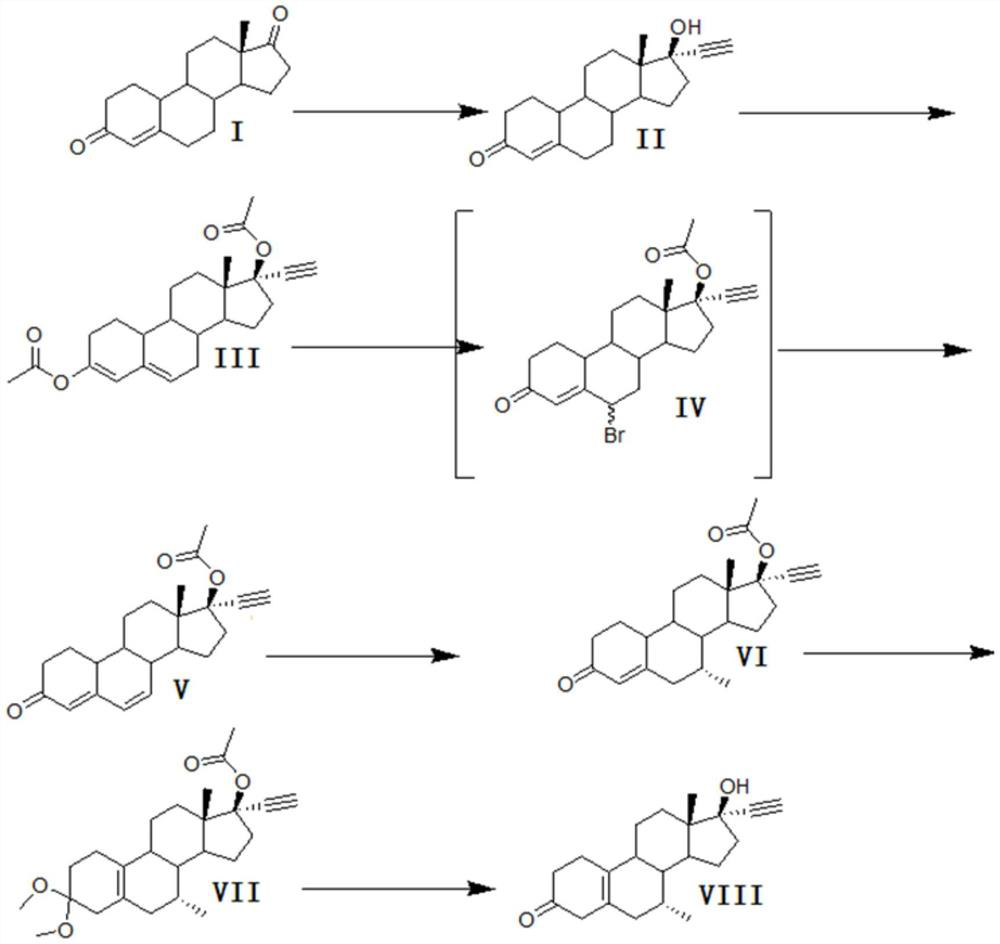
A kind of synthetic method of tibolone
A synthetic method, the technology of tibolone, applied in the field of synthesis of steroidal compounds, can solve the problems of unfavorable large-scale production operation and low yield, and achieve the effects of saving labor and energy costs, simple operation, and reducing raw material costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] A kind of synthetic method of embodiment 1 tibolone, described synthetic route is as follows
[0029]
[0030] 1) Alkynylation reaction:
[0031] 800kg of toluene, 20kg of potassium hydroxide, and 40kg of tert-butanol were dehydrated under normal pressure under reflux for 2 hours, cooled to 30°C, and a solution formed by dissolving 100kg of acid decarboxylate (I) in 200kg of toluene was added, and stirred for 10 minutes. Feed acetylene gas, start to detect TLC after 6 hours of ventilating, TLC analysis raw material reaction is complete, stop ventilating, add 200kg water to quench washing, separate layers. Concentrate under reduced pressure, add 100kg of ethanol, discharge, and dry to obtain 100kg of norethindrone (II), with a mass yield of 100%, detection by HPLC ≥ 99%, and single impurity ≤ 0.5%.
[0032] 2) Acylation reaction
[0033] Step 1) Add 200kg of acetic anhydride to 100kg of norethindrone (II) obtained, 100kg of pyridine, add 120kg of acetyl chloride dro...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com