Method for simultaneously determining arsenic and mercury in textiles while achieving microwave digestion
A microwave digestion and textile technology, applied in the field of heavy metal detection, can solve the problems of strong interference in the detection of arsenic and mercury, and the inability to detect arsenic and mercury at the same time.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
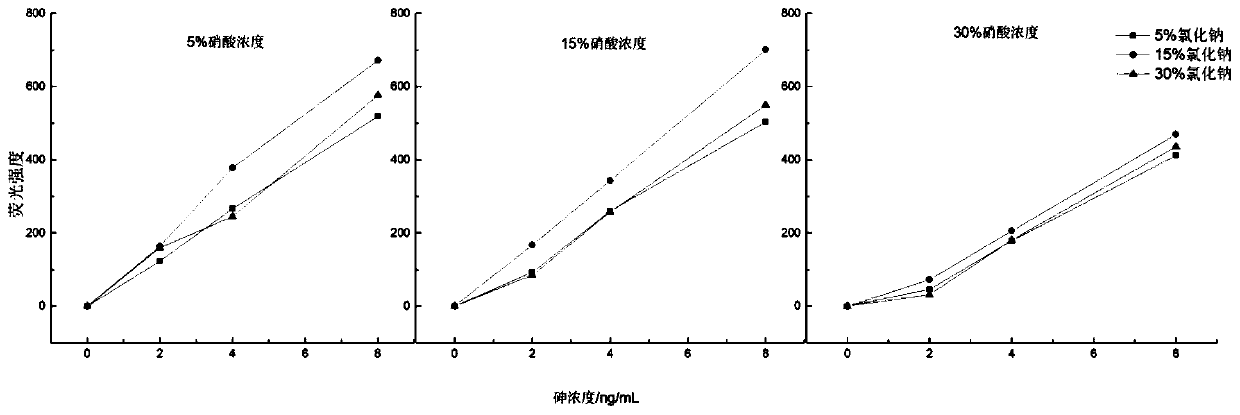
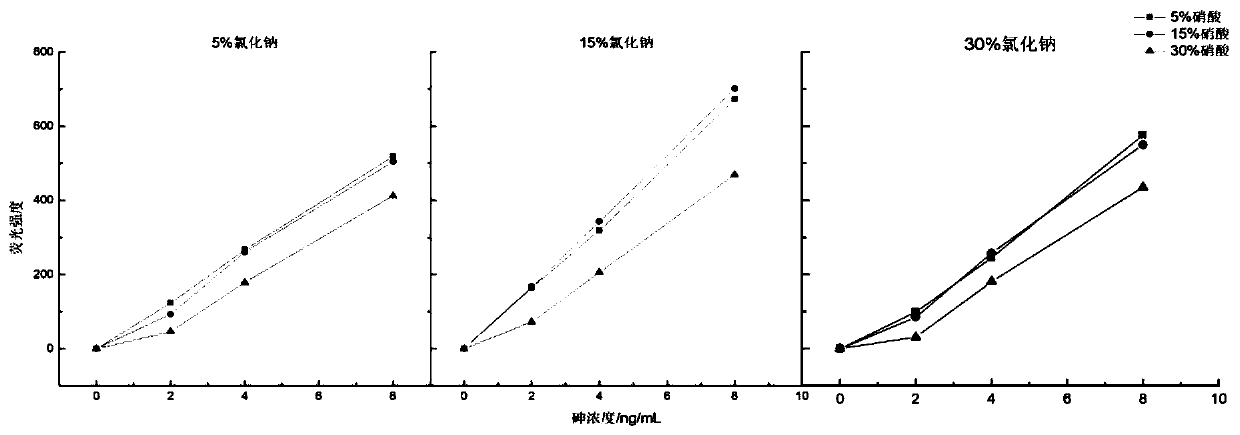
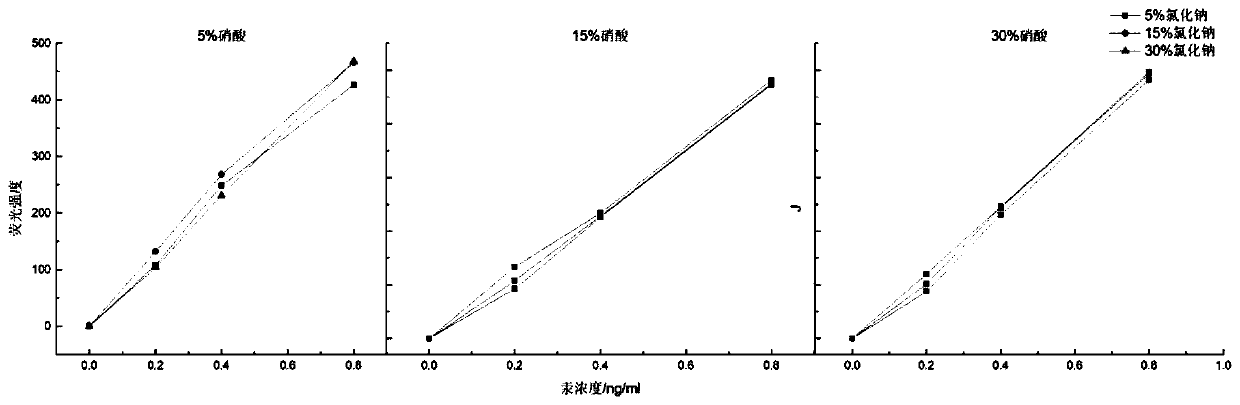
[0052] The selection of embodiment 1 solvent concentration
[0053] Simultaneous determination of arsenic and mercury in textiles requires that arsenic and mercury can coexist in the same solvent without interfering with each other. The present invention uses a mixed solution of nitric acid and sodium chloride with a certain concentration as the solvent for the coexistence of arsenic and mercury. According to the making method of the standard curve that arsenic and mercury mixed standard solution gained, adopt single factor variable analysis, take the volume concentration of the concentrated nitric acid in the standard solution and the mass concentration of sodium chloride as variable; According to table 1 respectively, different concentration The concentrations of sodium chloride and nitric acid were prepared with arsenic concentration: 0ng / mL, 2ng / mL, 4ng / mL, 8ng / mL; mercury concentration: 0ng / mL, 0.2ng / mL, 0.4ng / mL, 0.8ng / mL.
[0054] The fluorescence intensities of various...
Embodiment 2
[0061] Embodiment 2 thiourea, ascorbic acid system
[0062] Pentavalent arsenic cannot react with potassium borohydride to form AsH 3 , Ascorbic acid can convert 5-valent arsenic to 3-valent arsenic, and potassium borohydride can generate AsH 3 , thiourea has a stabilizing effect on ascorbic acid, ensuring the stability of arsenic during reduction, and thiourea interacts with ascorbic acid. This experiment adopts an orthogonal experiment with 3 factors and 4 levels, and considers the interaction between ascorbic acid and thiourea. In the case of different factors, the linear relationship between arsenic and mercury is analyzed, and the correlation coefficient of the linear relationship between arsenic and mercury is used as the evaluation result. Among them, the degree of freedom of factor A is 2, the degree of freedom of factor B is 2, the degree of freedom of factor C is 2, the degree of freedom of interaction between A and B is 4, and the total degree of freedom is 10, so ...
Embodiment 3
[0073] The optimization of embodiment 3 atomic fluorescence spectrometry conditions
[0074] 1. Experimental program design
[0075] Potassium borohydride reacts with acid to produce hydrogen gas and arsenic to form hydride, and arsenic atoms are produced at high temperature; mercury directly reacts with potassium borohydride to produce mercury atoms, and then arsenic and mercury atoms produce fluorescence under the hollow cathode lamp. , Mercury detection is critical. According to experimental analysis, sulfuric acid is used as a carrier to easily precipitate arsenic, and the decomposition product of nitric acid is easy to produce quenching. Hydrochloric acid has the best effect, so the present invention selects hydrochloric acid as a carrier. Hydrochloric acid reacts with potassium borohydride to generate hydrogen, and forms a hydride with trivalent arsenic , if the concentration is too low, the fluorescence intensity of arsenic and mercury will be weakened. High-concentrat...
PUM
| Property | Measurement | Unit |
|---|---|---|
| recovery rate | aaaaa | aaaaa |
| recovery rate | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com