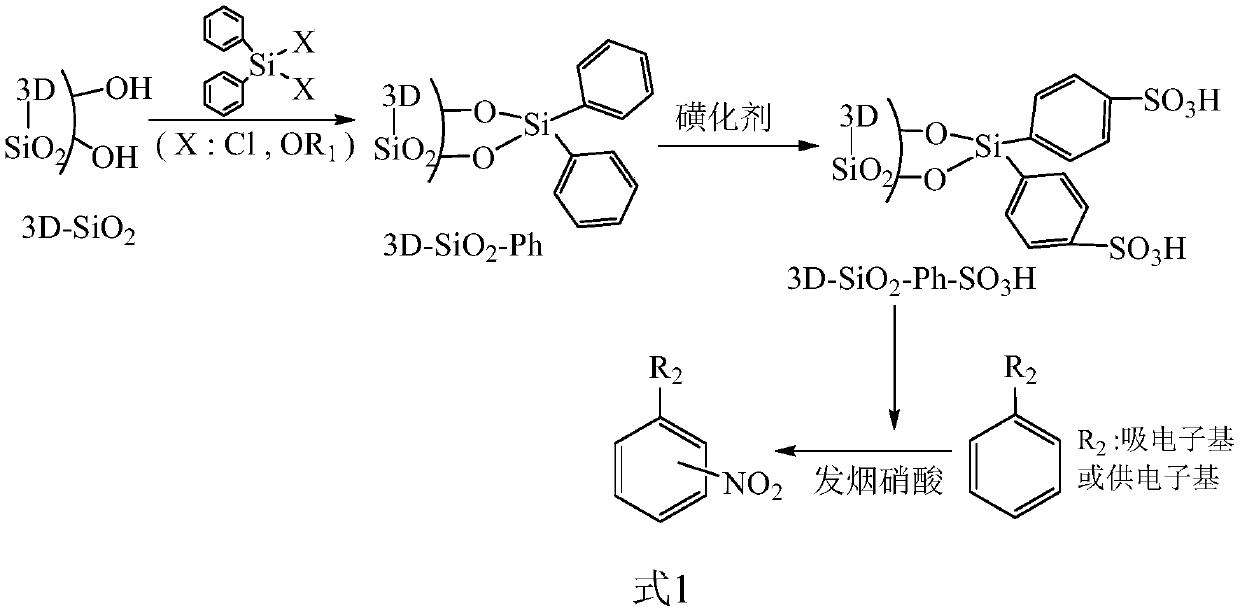
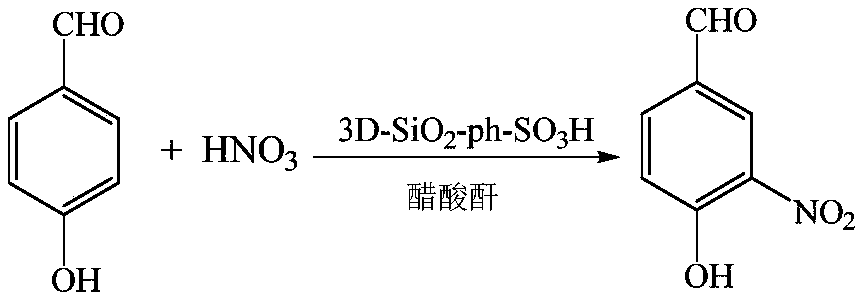
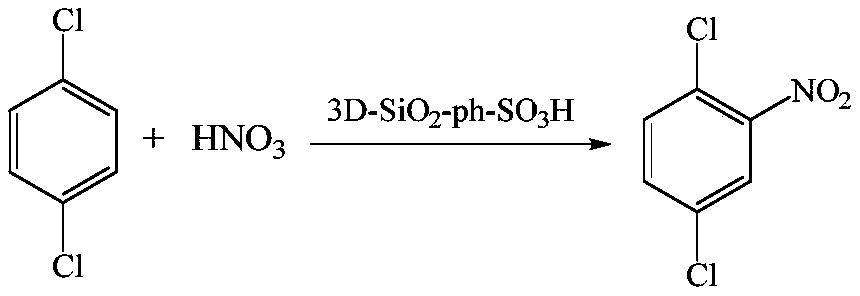High-acidity solid sulfonic acid catalyst for catalyzing aromatic compound nitrification, and preparation method
A technology of aromatic compounds and sulfonic acid catalysts, applied in the direction of organic compound/hydride/coordination complex catalysts, nitro compound preparation, physical/chemical process catalysts, etc. Small, unfavorable heat and mass transfer and other problems, to achieve the effect of good cycle performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] (1) Preparation of 3D-SiO 2 : Using P123 as a template, 3-methylbutanol as a pore-expanding agent, and ethyl orthosilicate as a gel-forming agent, the gel is generated under the catalysis of concentrated hydrochloric acid. The feeding weight ratio is: ethyl orthosilicate: P123: 3-methylbutanol: concentrated hydrochloric acid: water = 100: 40: 55: 75: 1500 (wt.), and the feeding amount of ethyl orthosilicate is 85g. The gel forming operation is: add triblock polyether and 3-methylbutanol into water to dissolve, then add concentrated hydrochloric acid and stir well. Then at 40°C, ethyl orthosilicate was uniformly added dropwise within 18 hours, and then reacted at 100°C for 20 hours. After suction filtration, washing with water, and calcination in air at 500°C for 5 hours, 19.7g of 3D-SiO was obtained 2 . Specific surface area 860m 2 / g, and the average pore diameter is 6.1 nm.
[0034] (2) The method of phenyl functionalization is: in toluene, use diphenyldimethoxys...
Embodiment 2
[0044] (1) Preparation of 3D-SiO 2 : Using P123 as a template, 3-methylbutanol as a pore-expanding agent, and ethyl orthosilicate as a gel-forming agent, the gel is generated under the catalysis of concentrated hydrochloric acid. The feeding weight ratio is: ethyl orthosilicate: P123: 3-methylbutanol: concentrated hydrochloric acid: water = 100: 50: 70: 90: 1750 (wt.), and the feeding amount of ethyl orthosilicate is 85g. The gel forming operation is: add triblock polyether and 3-methylbutanol into water to dissolve, then add concentrated hydrochloric acid and stir well. Then, tetraethyl orthosilicate was uniformly added dropwise within 35 to 12 hours, and then reacted at 90°C for 22 hours. After suction filtration, washing with water, and calcination in air at 550°C for 4 hours, 20.8g of 3D-SiO was obtained 2 . The specific surface area is greater than 888m 2 / g, the average pore diameter is 7.6nm.
[0045] (2) The method of phenyl functionalization is: in toluene, use d...
Embodiment 3
[0054] (1) Preparation of 3D-SiO 2 : Using F127 as a template, 3-methylbutanol as a pore-expanding agent, and ethyl orthosilicate as a gelling agent, the gel is generated under the catalysis of concentrated hydrochloric acid. The feeding weight ratio is: ethyl orthosilicate: F127: 3-methylbutanol: concentrated hydrochloric acid: water = 100: 60: 80: 110: 2000 (wt.), and the feeding amount of ethyl orthosilicate is 85g. The gel forming operation is: add triblock polyether and 3-methylbutanol into water to dissolve, then add concentrated hydrochloric acid and stir well. Then, tetraethyl orthosilicate was uniformly added dropwise within 24 hours at 25°C, and then reacted at 80°C for 24 hours. After suction filtration, washing with water, and calcination in air at 600°C for 3 hours, 19.2g of 3D-SiO was obtained 2 . The specific surface area is greater than 804m 2 / g, and the average pore diameter is 9.0 nm.
[0055] (2) The method of phenyl functionalization is: in toluene, u...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Specific surface area | aaaaa | aaaaa |
| Average pore size | aaaaa | aaaaa |
| Specific surface area | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com