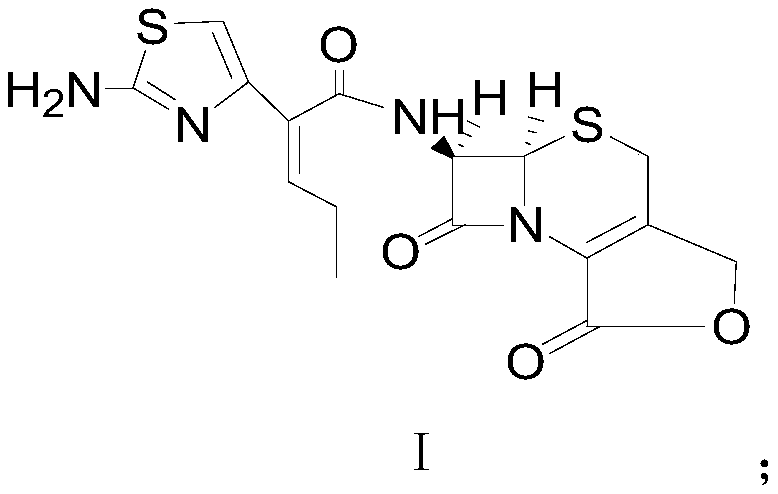
Preparation method of cefcapene lactone compound or hydrochloride thereof
A technology for cefcapine and compound, which is applied in the field of preparation of cefcapine lactone compound or its hydrochloride, can solve problems such as difficulty in extraction, achieve good yield and purity requirements, is beneficial to industrial application, and guarantees impurities Effects of Control and Purity Quality Analysis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
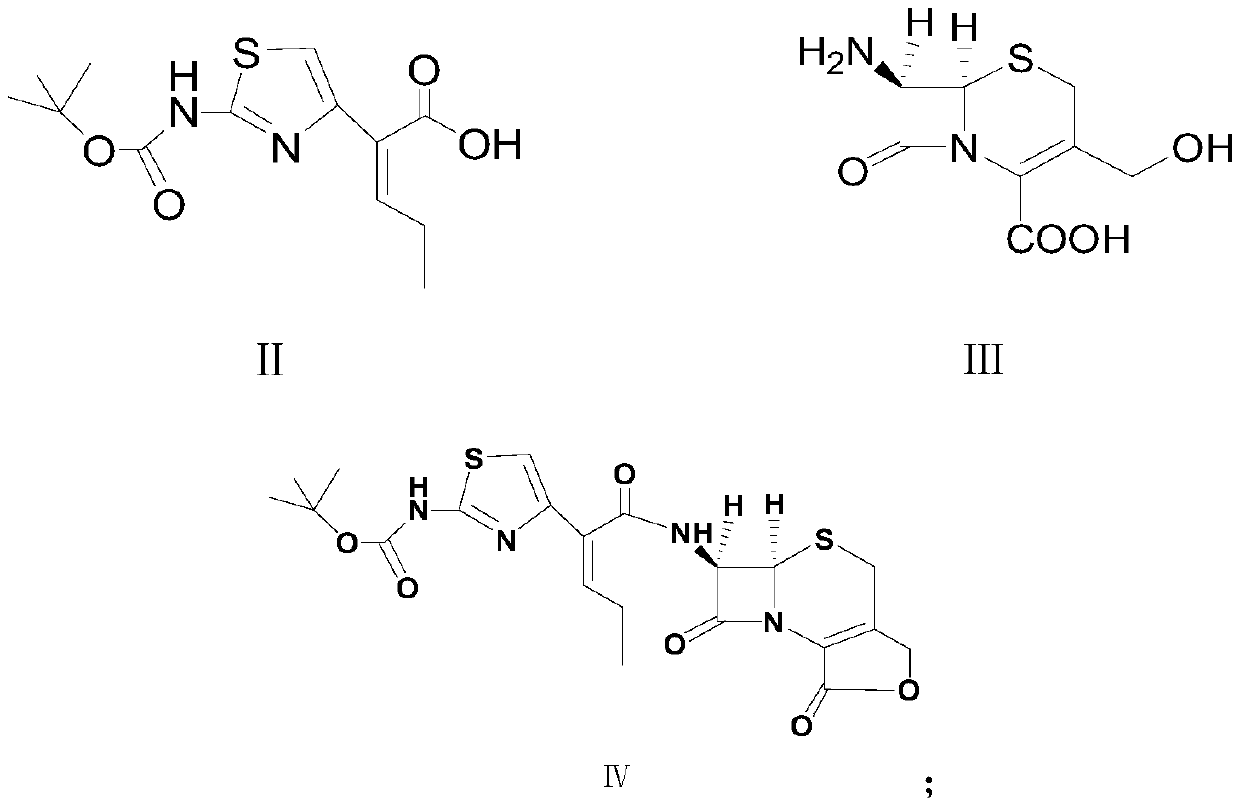
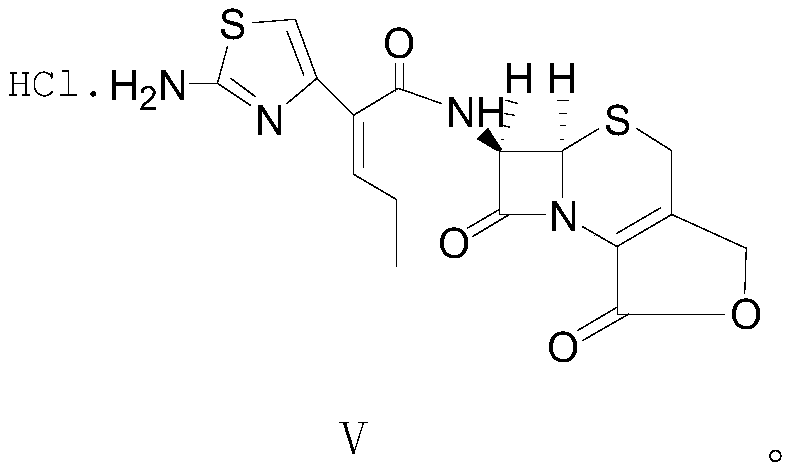
[0038] The formula V compound cefcarpine lactone hydrochloride structural formula of the present embodiment is as follows:
[0039]
[0040] The synthesis of above-mentioned cefcarpine lactone compound hydrochloride adopts the following method to obtain:
[0041] At room temperature, mix 30.2g (0.096mol, 1.2eq) (Z)-2-(2-tert-butoxycarbonylaminothiazol-4-yl)-2-pentenoic acid with 18.4g (0.08mol, 1eq) methylol Dissolve 7-aminocephalosporanic acid in 300mL of ethyl acetate, slowly cool down to 5-10°C, control the temperature, start to add 10.5g (0.104mol, 1.3eq) of triethylamine dropwise, after the drop, control the temperature at Stir at 5-10°C and carry out condensation reaction for 3 hours; after the condensation reaction is completed, add triethylamine dropwise to the reaction solution to adjust the pH value of the reaction solution system to 9.0-9.5. After the pH value is basically stable, control the temperature at Stir at about 15°C for sufficient lactonization reactio...
Embodiment 2
[0067] At room temperature, mix 37.7g (0.12mol, 1.5eq) (Z)-2-(2-tert-butoxycarbonylaminothiazol-4-yl)-2-pentenoic acid with 18.4g (0.08mol, 1eq) methylol Dissolve 7-aminocephalosporanic acid in 400mL of dichloromethane, slowly cool down to 5-10°C, control the temperature, start to add 10.5g (0.104mol, 1.3eq) triethylamine dropwise, after the drop, control the temperature at 0~5℃ and stirring for condensation reaction for 4 hours; after the condensation reaction is completed, add dropwise to the reaction solution, and then add dropwise triethylamine to the reaction solution, adjust the system pH of the reaction solution to 9.0-9.5, wait for the pH After the value is basically stable, control the temperature at about 15°C and stir for a full lactonization reaction for 3.0 hours. After the end of lactonization, carry out vacuum concentration to a certain volume to remove part of the solvent, and then slowly cool down to -10~-5°C for stirring Crystallize for 2 hours. After the cry...
Embodiment 3
[0071] At room temperature, mix 27.7g (0.088mol, 1.1eq) (Z)-2-(2-tert-butoxycarbonylaminothiazol-4-yl)-2-pentenoic acid with 18.4g (0.08mol, 1eq) methylol Dissolve 7-aminocephalosporanic acid in 400mL of ethyl acetate, slowly cool down to 5-8°C, control the temperature, start to add 12.1g (0.12mol, 1.5eq) of triethylamine dropwise, after the dropping, control the temperature at 0~5℃ and stirring for 3.5 hours for condensation reaction; after the condensation reaction is over, add triethylamine dropwise to the reaction solution to adjust the pH value of the reaction solution system to 9.0-9.5. After the pH value is basically stable, control the temperature at Stir at around 20°C for 3 hours for sufficient lactonization reaction. After the end of lactonization, carry out vacuum concentration to a certain volume to remove part of the solvent, then slowly cool down to -8~-5°C for 2.5 hours of stirring and crystallization, and crystallization After finishing, filter and wash the fi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com