Catalyst for preparing chlorine by oxidizing hydrogen chloride, preparation method and applications thereof
A catalyst, hydrogen chloride technology, applied in the direction of metal/metal oxide/metal hydroxide catalyst, preparation with chloride, physical/chemical process catalyst, etc., can solve the problems of poor activity, high process cost, poor stability, etc., to achieve Improve the activity, the process is simple and easy to operate, and the effect of increasing stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1~ Embodiment 16
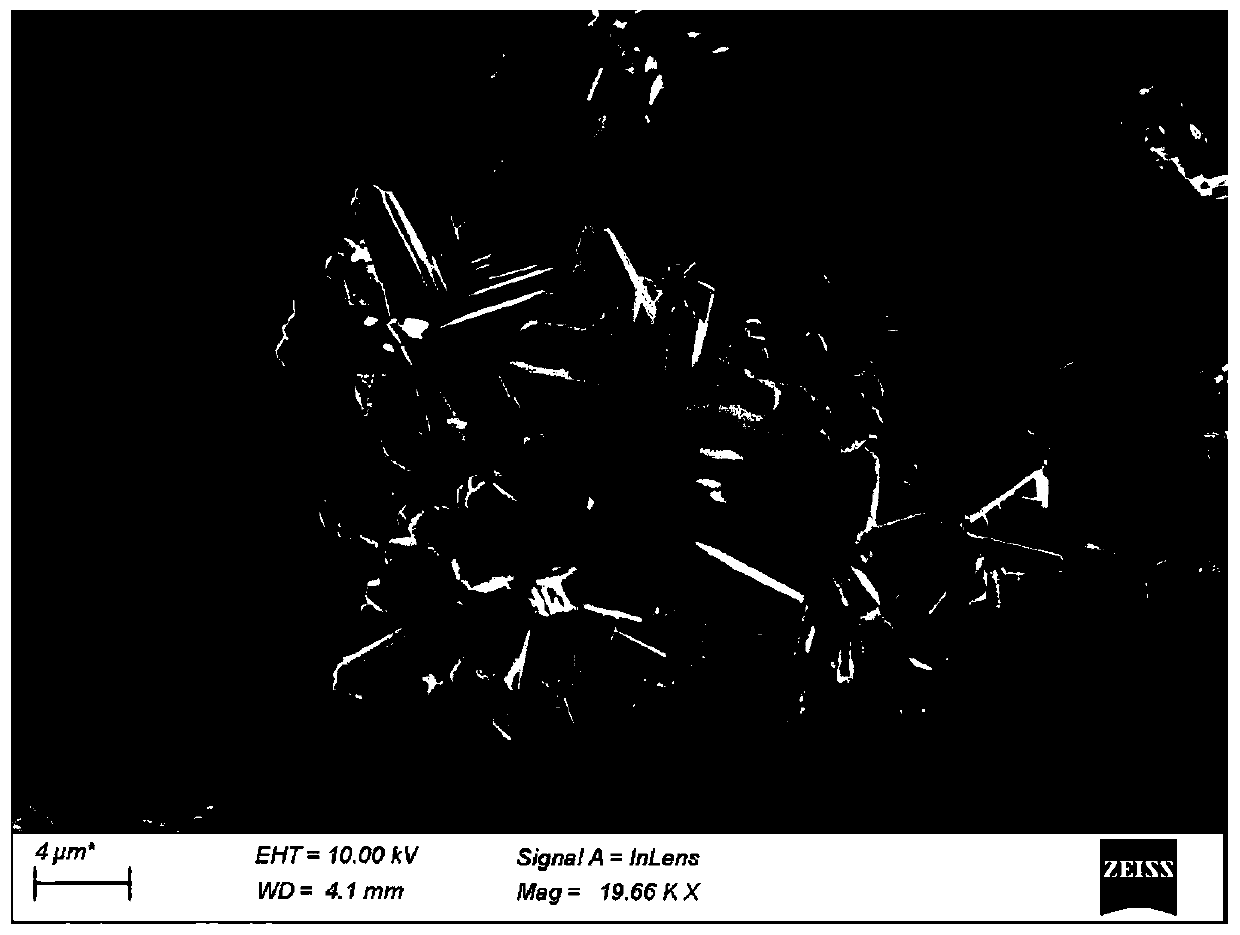
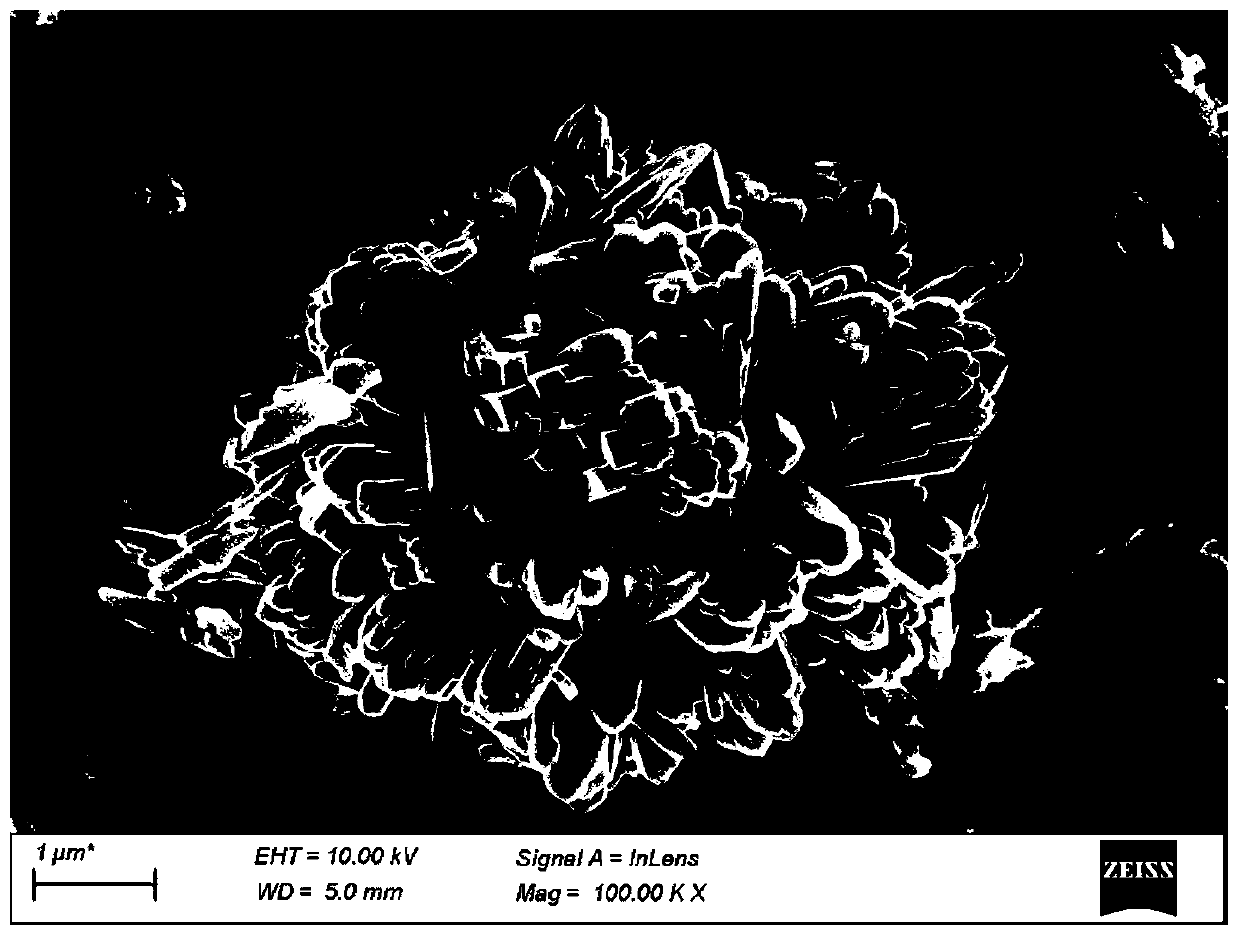
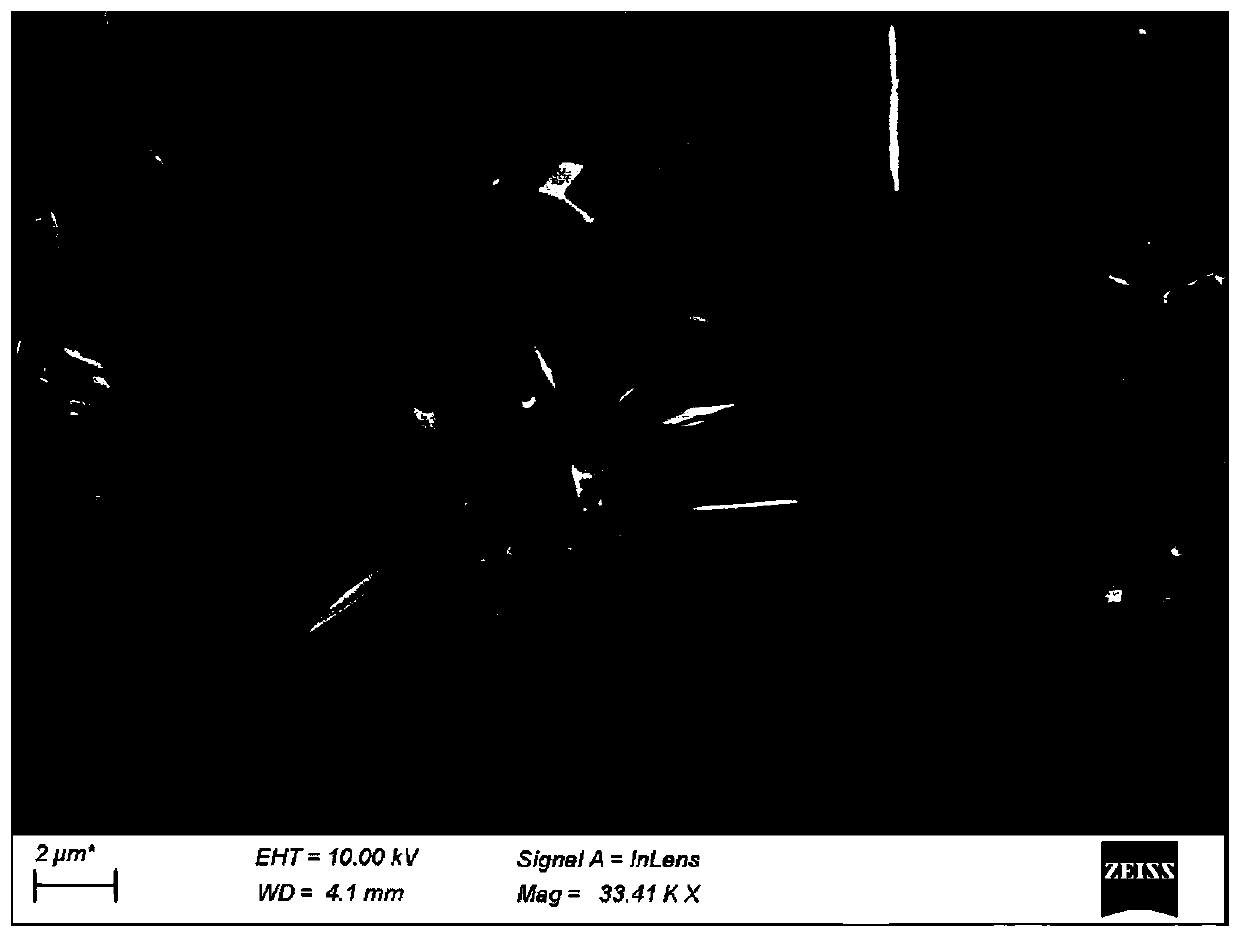
[0048] Take a certain amount of TiCl at room temperature 4 、RuCl 3 and NaF were dissolved in 0.1mol / L hydrochloric acid solution, and then the mixed solution was transferred to a polytetrafluoro-lined stainless steel reactor, crystallized overnight, and after the reactor was cooled, it was centrifuged at a speed of 5000r / min , and washed 4-5 times with ultrapure water, the obtained white sample was dried overnight in a vacuum oven at 100 ° C, and finally the sample was calcined in a muffle furnace for 4 hours to obtain RuO with sea urchin morphology 2 / TiO 2 catalyst.
[0049] The TiCl of embodiment 1~embodiment 16 4 、RuCl 3 And the dosage of NaF, the pH value of the crystallization solution, the crystallization temperature, the crystallization time and the calcination temperature are shown in Table 1 below.
[0050] Table 1. The process operating conditions of embodiment 1~embodiment 16
[0051]
[0052]
[0053] The RuO of the sea urchin morphology that embodimen...
Embodiment 16
[0056] HCl conversion and Cl of Example 16 2 Space-time yield (kg(Cl 2 ) / kg (catalyst) / h) as shown in Table 9.
Embodiment 17~ Embodiment 21
[0058] The preparation method that embodiment 17~embodiment 21 involves is identical with embodiment 14, and the difference between embodiment 17~embodiment 21 and embodiment 14 is that the reaction temperature of catalyst is different in application, and embodiment 17~embodiment 21 The reaction temperature is shown in Table 2. The HCl transformation rate and Cl of embodiment 17~embodiment 21 2 Space-time yield (kg(Cl 2 ) / kg (catalyst) / h) as shown in Table 9.
[0059] Table 2. The reaction temperature of embodiment 17~embodiment 21
[0060] Reaction temperature (°C) Example 17 330 Example 18 340 Example 19 360 Example 20 370 Example 21 380
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com