Auxiliary agent, preparation method of auxiliary agent and recrystallization method of laurolactam
A technology of cinnamic lactam and recrystallization, which is applied in the separation/purification of lactam, chemical instruments and methods, organic chemistry, etc., can solve the problems of cumbersome operation and achieve the effects of simple process, lower interface energy and low cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
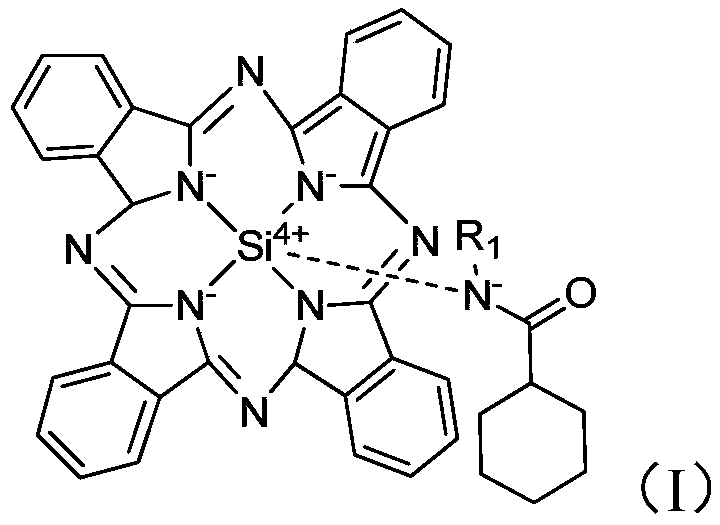
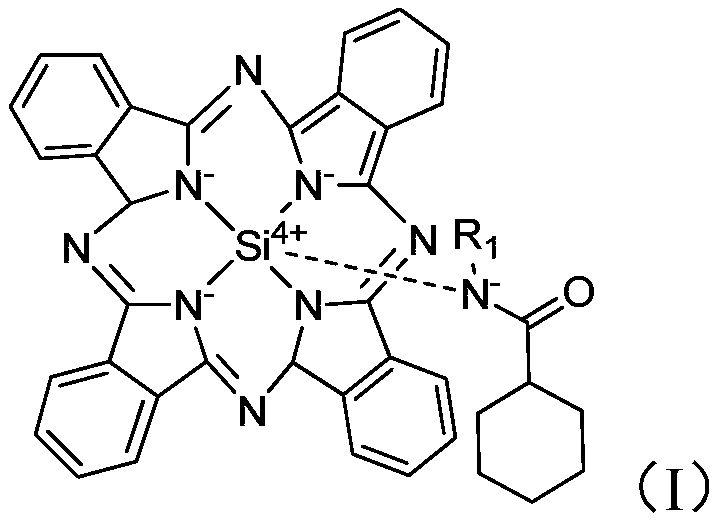
[0043] Auxiliary agent a is synthesized (structure is as formula (I), and R 1 =-CH 2 CH 3 ):
[0044] Weigh 35g of 1,3-diiminoisoindoline into a 250mL three-necked reaction bottle, which is connected with a thermometer and a condenser respectively, add 10g of silicon tetrachloride into the system, put it into a magnet, and put it in an oil bath Heat up to 210°C for reflux reaction, use a silica gel plate to monitor the reaction, and reflux for 1 hour;
[0045] Cool to 50°C, add 3g of ethylamine to the system, stir for 10min, then add 20mL of dichloromethane, 2mL of triethylamine (the molar ratio of 1,3-diiminoisoindoline to triethylamine is about 0.05) and 9g cyclohexanoyl chloride, continue to stir and react for 30min, separate and obtain auxiliary agent a 39g, yield 95%;
[0046] Elemental analysis of additive a: C, 70.36; H, 4.72; N, 18.46; O, 2.34; Si, 4.11;
[0047] 1 H NMR (400MHz, DMSO-d 6 ):δ1.22(-CH 3 ),1.56~1.81(-CH 2 -,), 2.38(-CH), 3.24(-CH 2 -), 5.56(2H)...
Embodiment 2
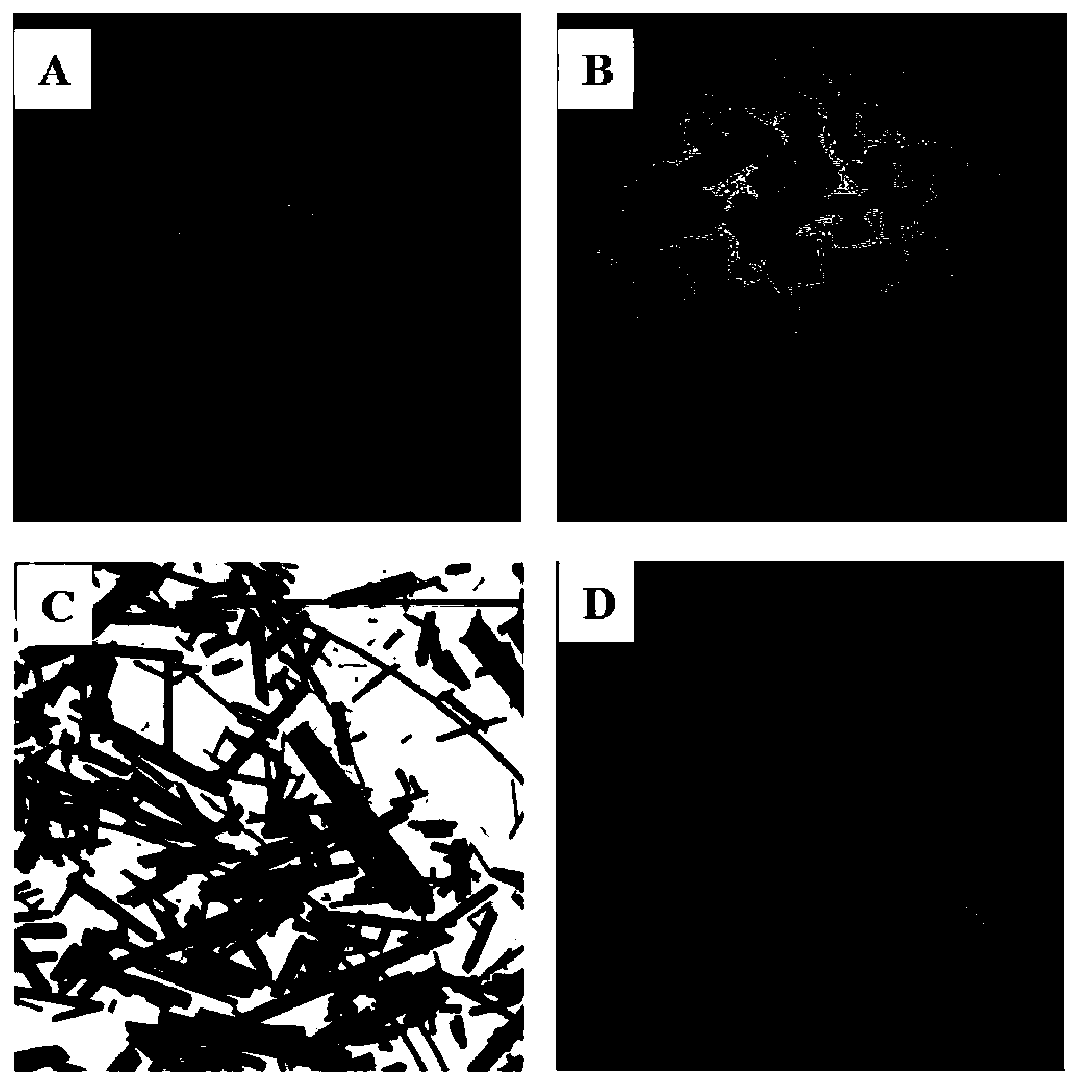
[0052] The difference with Example 1 is that in the recrystallization process, the auxiliary agent used is replaced by auxiliary agent b (the difference with auxiliary agent a is that R in formula I 1 =CH 3 ), other feeding intake and experimental conditions in the recrystallization process are all consistent with embodiment 1, no particle coalescence occurs in the recrystallization process, and the yield of the recrystallization process is 93.3% after analysis, and the purity is 99.4wt%. Electron microscope pictures are attached figure 1 As shown in Figure B;
[0053] The synthesis process of auxiliary agent b is basically the same as that of auxiliary agent a in Example 1, the only difference is that ethylamine is replaced by methylamine; the structural detection information of auxiliary agent b is as follows:
[0054] Elemental analysis of the obtained additive b: C, 70.36; H, 4.72; N, 18.46; O, 2.34; Si, 4.11.
[0055] 1 H NMR (400MHz, DMSO-d 6 ): δ1.53~1.73(-CH 2 -)...
Embodiment 3
[0057] The difference from Example 1 is that during the recrystallization process, 10 g of auxiliary agent a was used, and no particle coalescence occurred during the recrystallization process. The yield of the recrystallization process was analyzed to be 87.9%, and the purity was 99.0 wt%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com